Figure 4.
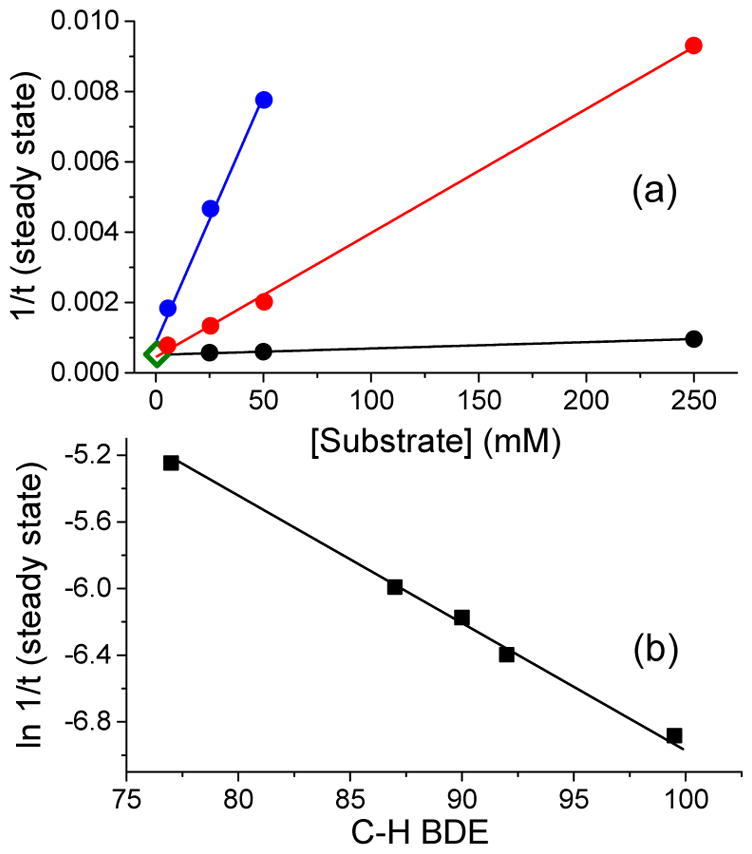
(a) Plot of the inverse length of the steady state duration of 3a* (460 nm) as a function of [cyclohexadiene] (blue), [cyclohexene] (red), and [1-octene] (black) in the 1a* (1 mM)/H2O2 (20 mM)/AcOH (200 mM)/substrate mixture in CH3CN at −40 °C. The point represented by the open diamond at the bottom left corner corresponds to the inverse length of the steady state duration of 3a* in the absence of any substrate. (b) Correlation between the natural log of the inverse length of the steady state for 3a* (460 nm) as a function of C–H bond dissociation energy in the 1a* (1 mM)/H2O2 (20 mM)/AcOH (200 mM)/substrate (250 mM) reaction mixture in CH3CN at −40 °C. Substrates used are from left to right cyclohexadiene, ethylbenzene, toluene, tetrahydrofuran, and cyclohexane.
