Abstract
Mismatch negativity (MMN) to deviant stimuli is robustly smaller in individuals with chronic schizophrenia compared with healthy controls (Cohen’s d > 1.0 or more), leading to the possibility of MMN being used as a biomarker for schizophrenia. However, there is some debate in the literature as to whether MMN is reliably reduced in first-episode schizophrenia patients. For the biomarker to be used as a predictive marker for schizophrenia, it should be reduced in the majority of cases known to have the disease, particularly at disease onset. We conducted a meta-analysis on the fourteen studies that measured MMN to pitch or duration deviants in healthy controls and patients within 12 months of their first episode of schizophrenia. The overall effect size showed no MMN reduction in first-episode patients to pitch-deviants (Cohen’s d < 0.04), and a small-to-medium reduction to duration-deviants (Cohen’s d = 0.47). Together, this indicates that pitch-deviant MMN is not a candidate biomarker for schizophrenia prediction, while duration-deviant MMN may hold some promise, albeit nearly a third as large an effect as in chronic schizophrenia. Potential causes for discrepancies between studies are discussed.
Keywords: mismatch negativity, schizophrenia, first-episode, pitch, duration, effect size
Introduction
Individuals with schizophrenia demonstrate sensory and cognitive deficits that appear to worsen after the first episode of psychosis. There are numerous reports of structural and functional neurological deficits that correlate with disease course,1-7 highlighting the need for a biomarker that can help identify those who are at risk for developing schizophrenia. Identifying at-risk individuals before their first psychotic break offers the opportunity for early pharmacological and/or behavioral intervention, potentially preventing the postbreak neurological deficits.8 Cognitive and psychosocial intervention at first episode has improved symptoms, functioning, and hospitalizations in these patients,9,10 even 10 years after the intervention began,11 and may have greater benefits prior to first-episode.
Mismatch negativity (MMN) is one potential electrophysiological biomarker for early identification. Biomarkers are defined to include, “ … biological characteristics that can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention.”12 We have previously argued that MMN is a potential biomarker for disease progression and as an outcome measure for pharmacological intervention.7 However, many studies have suggested that MMN be a biomarker for the presence of the disease at the first break for schizophrenia. The purpose of this article is to assess this. MMN is an event-related potential (ERP) that appears with the presentation of deviant stimuli.13 For example, single tones that differ in pitch or duration among repeated standard tones elicit an MMN. Source analysis of MMN in EEG and magnetoencephalography signal localizes MMN to auditory cortex,14-16 with an additional later source from prefrontal cortex.14,17
MMN also has some clinical utility.18,19 Individuals with chronic schizophrenia exhibit reduced MMN amplitudes, 0.94 SDs smaller for pitch deviants and 1.23 SDs smaller for duration deviants compared with healthy controls.20 MMN correlates with reductions in gray matter,7 correlates with disease severity and cognitive dysfunction,21 and impaired social functioning.22 MMN reductions may reflect deficits in NMDA receptors, 23-26 which are abnormal in schizophrenia,27-30 suggesting a neurological mechanism for MMN that can be tracked through the progression of the disease. MMN deficits also appear to be more severe in schizophrenia compared to individuals with bipolar disorder31 or Alzheimer’s disease. 32
MMN is appealing as a biomarker for disease presence because of the relatively easy, quick, and inexpensive methods of measurement. MMN can be recorded during a short EEG session and is measured passively. This is particularly helpful when working with clinical populations. One use for a biomarker is to help detect those who are at-risk for developing schizophrenia before their first-psychotic break.12 However, for a biomarker to be useful for early detection purposes, it must also be abnormal early in the disease course. There is current controversy whether MMN to simple physical characteristics is reduced at the first episode of schizophrenia, or is essentially unaffected, with deficits emerging with disease course. Although several studies have found reduced duration MMN in first-episode schizophrenia patients, other studies have found no significant reduction in duration or pitch MMN, with the occasional study even reporting nominally greater MMN amplitudes compared with healthy controls. A biomarker should be sensitive in distinguishing between positive (schizophrenia) and negative (nonschizophrenia) cases, that is, there should be little overlap in population distributions. Hence, for MMN to serve as a viable biomarker of disease presence, it should be generally reduced in the majority of first episode cases, with a relatively large effect size. The large effect size observed in chronic schizophrenia (d = ~1.0) means that approximately 61.7% of the samples overlap. A medium effect size (d = 0.5) means that 80% of the groups overlap, and a small effect size (d = 0.2) means that 92% of the groups overlap.
To assess the existing empirical data regarding MMN reduction in first-episode patients, a meta-analysis was conducted. The term first-episode psychosis is diagnostically ambiguous and includes various diagnoses with the schizophrenia-spectrum (eg, schizophrenia, schizophreniform, delusional disorder, psychosis not otherwise specified, etc) and within the affective psychosis spectrum (eg, major depressive disorder with psychosis, bipolar disorder with psychosis, etc). Therefore, studies that used only schizophrenia-spectrum participants were analyzed separately from mixed psychosis samples. Studies that did not account for or equate participants on premorbid IQ or years of education, potential confounding variables, are indicated. Analyses were conducted separately for pitch and duration-deviant MMN. Several studies report MMN from both deviant-types and so appear in both analyses. Follow-up meta-analyses include mixed schizophrenia-and affective-spectrum samples.
Methods
Literature Search
A PubMed search was conducted using the keywords “mismatch negativity,” “first-episode,” and “schizophrenia,” and returned 23 published article. One was a review article,33 another was a meta-analysis in chronic patients,30 and a third article was a commentary for MMN in converters versus non-converters.34 Of the remaining 20 articles, one measured MMN in prodromal patients,36 another in individuals who were ultra-high-risk of developing schizophrenia,36 and another was on schizotypal personality disorders.37 One article did contain first-episode patients, but combined MMN responses with those from individuals with chronic schizophrenia.38 Sixteen studies remained that were further assessed. Another 7 studies were found via reference sections, and one submitted (but not yet published) study was included. In total, 24 first-episode psychosis MMN articles were found.
Inclusion and Exclusion Criteria
There were 3 exclusion criteria. First, patients were considered as being in their first episode if they completed the MMN task within 12 months of first admission to hospital and had no more than 1 psychotic episode in their lifetime. This led to 3 articles being rejected.39-41 In addition, 1 article mixed first- and second-episode schizophrenia-spectrum patients.42 Second, studies that included patients with affective disorders and did not analyze their MMN separately from those with schizophrenia-spectrum diagnoses were excluded, resulting in 5 studies being excluded from analysis.43-47 The SDs from 1 article could not be ascertained for the first-episode or control group.48 The remaining 14 studies were included in the analysis,7,49-61 and the results from these studies are summarized in Table 1.
Table 1.
Number of Participants (N), Means, and Standard Deviations (SD) for First-Episode (FE) and Healthy Control (HC) Groups, and Effect sizes for Each Study Included in the Analysis.a
| Study | Task | N FE/HC | Mean FE | Mean HC | SD FE | SD HC | Effect Size |
IQ/Education- Matched? |
|---|---|---|---|---|---|---|---|---|
| Salisbury (2002)60 | Pitch | 21/27 | −3.55 | −3.76 | 2.50 | 2.00 | 0.09 | Yes |
| Valkonen-Korhonen (2003)59 |
Pitch | 25/29 | −1.75 | −1.82 | 1.52 | 1.26 | 0.05 | Included as covariate |
| Umbricht (2006)57 | Pitch | 26/39 | −1.15 | −1.61 | 0.38 | 1.52 | 0.31 | No |
| (College subsample)57 | Pitch | 12/39 | −1.77 | −1.61 | 0.46 | 1.52 | −0.10 | Yes |
| (No college subsample)57 | Pitch | 14/39 | −0.54 | −1.61 | 0.29 | 1.52 | 0.67 | No |
| Salisbury (2007)7 | Pitch | 20/32 | −3.84 | −3.82 | 2.54 | 2.67 | −0.01 | Yes |
| Magno (2008)56 | Pitch | 12/27 | −2.80 | −2.33 | 1.10 | 1.40 | −0.35 | NR |
| Devrim-Ucok (2008)55 | Pitch | 30/34 | −3.90 | −3.90 | 2.40 | 2.80 | 0.00 | Yes |
| Bodatsch (2011)54 | Pitch | 33/67 | −2.82 | −2.95 | 1.30 | 1.15 | 0.11 | No |
| Nagai (2013)51,b | Pitch | 20/22 | NR | NR | 0.97 | 1.15 | 0.33 | Yes |
| Salisbury submitted61 | Pitch | 29/40 | −1.90 | −1.70 | 2.20 | 1.70 | −0.10 | Yes |
| Umbricht (2006)57 | Duration | 26/39 | −0.94 | −1.19 | 0.33 | 0.99 | 0.25 | No |
| (College subsample)57 | Duration | 12/39 | −1.69 | −1.19 | 0.46 | 0.99 | −0.43 | Yes |
| (No college subsample)57 | Duration | 14/39 | −0.19 | −1.19 | 0.20 | 0.99 | 0.96 | No |
| Oades (2006)58 | Duration | 28/22 | −1.10 | −2.10 | 0.90 | 1.10 | 1.00 | No |
| Magno (2008)56 | Duration | 12/27 | −3.21 | −2.71 | 1.98 | 2.12 | −0.24 | NR |
| Hermans (2010)47 | Duration | 17/17 | −3.40 | −4.90 | 1.50 | 2.40 | 0.73 | No |
| Bodatsch (2011)54 | Duration | 33/67 | −2.49 | −3.04 | 1.33 | 1.15 | 0.45 | No |
| Kaur (2011)53 | Duration | 18/18 | −3.70 | −6.40 | 1.70 | 1.90 | 1.47 | No |
| Kaur (2012)42 | Duration | 20/20 | −3.40 | −6.30 | 2.00 | 2.10 | 1.39 | No |
| Atkinson (2012)46 | Duration (long deviant) | 10/20 | −1.00 | −1.97 | 0.86 | 0.50 | 1.48 | No |
| Atkinson (2012)46 | Duration (short deviant) | 10/20 | −0.76 | −1.37 | 0.95 | 0.35 | 0.97 | No |
| Hsieh (2012)52 | Duration | 32/56 | −0.94 | −1.37 | 0.84 | 0.89 | 0.49 | Yes |
| Nagai (2013)51 | Duration | 20/22 | NR | NR | 1.24 | 1.78 | 0.78 | Yes |
| Higuchi (2013)43 | Duration | 20/20 | −5.60 | −7.90 | 1.70 | 1.10 | 1.61 | NR |
| Higuchi (2014)44 | Duration | 19/19 | −5.40 | −7.40 | 1.90 | 1.40 | 1.67 | NR |
| Mondragon-Maya (2013)50 | Duration | 20/23 | −1.50 | −1.70 | 1.08 | 1.30 | 0.16 | Yes |
| Solfs-Vivanco (2014)49 | Duration | 20/23 | −1.46 | −2.40 | 0.72 | 0.89 | 1.14 | Yes |
| Salisbury submitted61 | Duration | 29/40 | −2.20 | −2.40 | 1.90 | 1.70 | 0.11 | Yes |
Abbreviation: NR, not reported.
Studies that matched for premorbid IQ and/or years of education indicated.
Nagai et al (2013)51 reported effect sizes for MMN differences between patients and controls but not mean MMN amplitudes.
Calculations
Unbiased Cohen’s effect size (d)62 for each study was calculated using formula (1). The variance of the unbiased effect sizes (σ2(d)) was calculated using formula (2), and used to calculate the average effect size (d+) (formula 3). All formulae are from Hedges and Olkin.63 Size of effect was interpreted using the guidelines presented by Cohen62: d < 0.2 is a negligible effect size, d > 0.2 is a small effect size, d > 0.5 is a medium effect size, d > 0.8 is a large effect size.
Formula 1: Calculations for unbiased effect size for each study used. N = number of observations; SD = standard deviation; FE = first episode; C = controls; i = each study.
Formula 2: Used to calculate the variance of the unbiased effect sizes. N = number of observations; di = unbiased effect size calculated using formula (1); FE = first episode; C = controls; i = each study.
Formula 3: To calculate the average effect size. di = unbiased effect size from formula 1; = unbiased variance from formula 2; k = all studies; i = each study.
Results
From the initial 24 studies reporting MMN in first-episode schizophrenia-spectrum, 14 met the 3 criteria mentioned above. All studies measured MMN from either a single tone that differed in either pitch or duration. Five studies measured MMN to pitch and duration deviants and so were included in both analyses.51,54,56,57,61
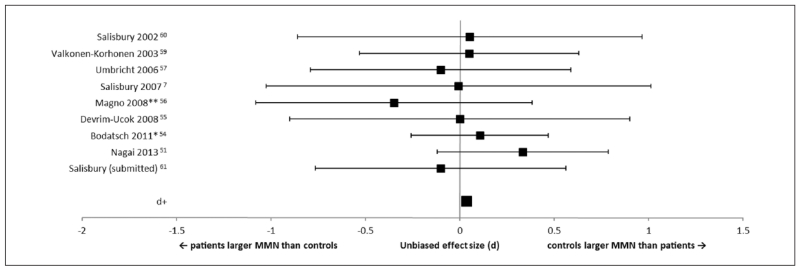
Nine studies reported MMN to pitch-deviants (Figure 1). There was a very small (negligible) effect size (d+ = 0.04) demonstrating that that there was no difference between first-episode patients and healthy controls on pitch-deviant MMN Seven studies controlled for IQ or years of education. When these studies were analyzed separately, the overall effect size was still negligible (d+ = 0.05).
Figure 1.
Forest plot of effect sizes for studies measuring mismatch negativity (MMN) to pitch-deviants. Error bars are 95% confidence intervals. The average effect size (d+) is at the bottom of the graph. **Studies that did not report IQ or years of education. *Studies that reported a significant difference in IQ and/or years of education between first-episode and control groups.
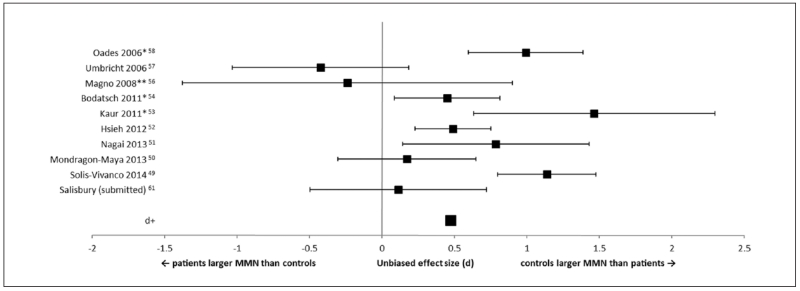
Eleven studies reported MMN to duration-deviants (Figures 2 and 3). There was a small to medium effect size (d+ = 0.47) for first-episode patients producing smaller duration-deviant MMN compared with controls. Only 6 out of the 11 studies controlled for IQ or years of education, and these 6 produced only a small effect size overall (d+ = 0.36).
Figure 2.
Forest plot of effect sizes for studies measuring mismatch negativity (MMN) to duration-deviants. Error bars are 95% confidence intervals. The average effect size (d+) is at the bottom of the graph. **Studies that did not report IQ or years of education. *Studies that reported a significant difference in IQ and/or years of education between first-episode and control groups.
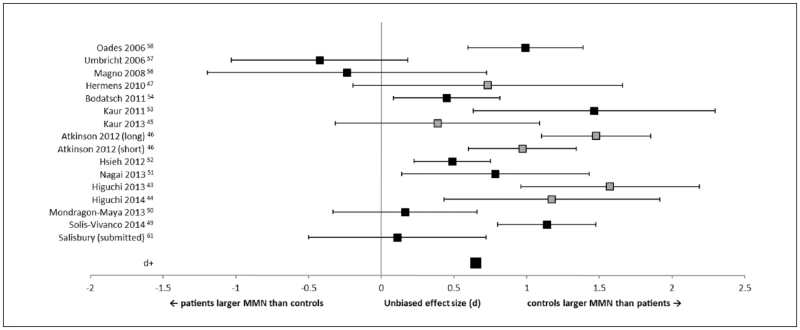
Figure 3.
Forest plot of effect sizes for studies measuring mismatch negativity (MMN) to duration-deviants, including studies with mixed schizophrenia spectrum and affective psychosis diagnoses. Error bars are 95% confidence intervals. The average effect size (d+) is at the bottom of the graph. Gray squares are the studies that contained mixed diagnosis samples.
Four studies included individuals with affective disorder in their first-episode psychosis groups.43-47 All measured duration-deviant MMN. Effect size was recalculated to include these studies. There was an increase in overall effect size (d+ = 0.65), although still only about half as large as the effect in chronically ill schizophrenia. However, 3 of these studies used a subgroup of the same participants,45,47,53 and an additional 2 studies also used the same participants.43,44 When studies were limited to the largest samples from each group,44,47,53 the effect size was reduced (d+ = 0.52).
Discussion
Comparison of studies measuring MMN reduction in first-episode schizophrenia-spectrum patients showed a negligible effect size of 0.04 SD for MMN to a pitch-deviant and a small to medium effect size of 0.47 SD for MMN to a duration-deviant. Effect sizes for MMN reductions in patients with chronic schizophrenia were around 1 SD compared with controls19 suggesting that the MMN deficit increases with the progression of the disease.
Umbricht and Krljes20 reported significant correlations between pitch MMN and duration of disease, and so it is likely that, at least for responses to pitch-deviants, MMN tracks the degenerative process associated with schizophrenia, and may not be affected until later stages of the disease. Indeed, 2 of the studies included in the analysis followed up with patients to measure their pitch MMN after their first psychotic episode. Two studies7,55 reported MMN between 1 and 18 months later and found that MMN was significantly smaller in patients compared with controls at retest, despite no significant difference in MMN during their first-episode of psychosis.
It is possible that MMN to duration-deviants does not track disease course and is (on average) abnormal at first-episode. However, with respect to using MMN as a biomarker for those at risk of developing schizophrenia, small reductions in MMN amplitude compared with the “norm” are unlikely to be detected at the individual level and so are unlikely to be helpful in identifying at-risk individuals. For chronic patients, an effect size of 1.23 indicated that only 53.9% of the populations overlap; for first episode patients with an effect size of 0.47, 81.4% of the populations overlap.
There is the possibility that the studies cited here are subject to sampling bias: The patients who are the sickest and have the worst MMN, typically return to hospital in the future and become the new chronic schizophrenia sample. This may explain the large MMN reductions in chronic patients compared with first-episode patients. While this is a valid concern, the few patients who have followed first-episode patients longitudinally have reported worsening MMN at follow-up7,45,55 suggesting that the small effect sizes are not due to sampling bias. Both of these studies measured pitch MMN and so it is possible that duration MMN does not track disease progression. Duration MMN reductions have been reported in schizophrenia patients and their first-degree relatives (without schizophrenia), suggesting that duration MMN may be a better endophenotype for schizophrenia.64 Longitudinal studies measuring duration MMN would be able to address this concern.
One of the moderators for MMN reductions in first-episode patients could be premorbid IQ or years in education. The concern is that clinical populations as a whole tend to have lower IQ and fewer years in education (likely as a consequence of their condition). However, MMN reductions in chronic schizophrenia to pitch, duration, and intensity deviants have been shown to correlate with years in education, and that the patients were more likely to have had fewer years in education compared with controls.65 One of the studies involved in the meta-analysis57 reported smaller MMN in first-episode patients, but when the first-episode group was divided into those who had finished college and those who had not, those who had attended college exhibited even larger amplitudes than controls. Matching groups on years of education may be overcorrecting, as we know that psychosis interrupts schooling so that individuals cannot finish college or high school. Matching for full-scale IQ likely overestimates premorbid IQ. However, some tests are more sensitive to psychosis such as semantic/overlearned knowledge. Matching on “hold-variables” avoids complementary problems of confounding low intellectual functioning with disease process (for further discussion of IQ see Salisbury et al61). It is interesting that 4 of the studies with the largest individual effect size in duration MMN reported patients having poorer premorbid IQ or completed fewer years in education compared with controls (Figure 2). Indeed, effect sizes for the duration MMN decreased from d = 0.47 to 0.36 when IQ or education was accounted for. At this small effect size, 85.7% of the groups overlap. It is possible that the patients who have a premorbid IQ similar to controls are at the higher end of the functioning scale and may not represent the schizophrenia population as a whole.
In addition, several studies reported MMN from a group of patients with psychosis, consisting of schizophrenia-spectrum and affective-psychosis individuals.43-45,47,53 It should be noted that a subset of participants were common across several of these articles,43-45,47,53 and so the results of these studies are not independent of each other, and may be inflating the effect size. The disease course of affective-psychosis may differ from schizophrenia making it difficult to ascertain the specificity of MMN reductions in early-course schizophrenia. For example, one of the studies involved in the meta-analysis7 reported larger MMN reductions longitudinally in schizophrenia-spectrum patients, but not in bipolar patients, compared with controls. In addition, there was a significant relationship between MMN amplitude and volume in left Heschl’s gyrus in schizophrenia-spectrum patients, but not in chronic bipolar patients or controls. Other studies have found normal MMN amplitudes in bipolar patients compared with controls,35,66 suggesting that the MMN may not be reliably abnormal in bipolar disorder as it is in chronic schizophrenia.
There are several potential confounds regarding studies with first-episode patients; the main one being the effect of medication. In general, there do not appear to be any effects of antipsychotic medication on MMN,67-69 but there is some inconsistency across studies.70 It should also be noted that these studies focused on chronic patients who had already been on medication for a substantial period of time. Medication may have a different effect on MMN in those who were previously naïve to antipsychotic medication. Even within the current analysis, there is inconsistency between the studies on whether medicated,44,47,51,61,63 unmedicated,49,50,54,59 or were a mixture of medicated and unmedicated patients,7,42,43,45,48,53,56,58,60,61 and 2 studies did not report medication status.46,52 Research on medication naïve individuals is needed, although longitudinal examination is unlikely, given the ethical and moral need to treat psychosis as early as possible.
Another factor is the amount of time between the onset of the first episode and measuring MMN. Across the studies assessed here, there is variability in how quickly the patients were tested when they reached the hospital. We have attempted to restrict the analysis to studies that measured MMN within 12 months of the first episode, the commonly used operational definition of first episode, but by 12 months substantial neurological changes may have already taken place. In addition, the duration of untreated psychosis may play a role in how abnormal the MMN is. If medication is able to stop or slow down the neurological decay associated with schizophrenia (at least temporarily), then those who were treated relatively quickly after their true first break may have better MMN than those who had not. Studies that are able to measure MMN as soon as the patient is admitted to hospital for their first episode will be able to address this issue.
Despite the marked MMN reductions in patients with chronic schizophrenia, there does not appear to be a strong deficit at first episode. Pitch MMN was clearly not reduced at first schizophrenic break. However, duration MMN showed a small to medium effect size. Perhaps in combination with other biomarkers duration MMN may have predictive value. Whilst this meta-analysis throws into question the use of MMN as a predictive biomarker in isolation, MMN can still be a useful indicator for pharmacological outcome71 and disease progression.7 The purpose of this meta-analysis was to assess MMN as a biomarker of disease presence. In summary, there is no consistent evidence for a marked deficit in pitch MMN in first-episode schizophrenia-spectrum patients, while duration MMN may show a small to medium effect size.
Acknowledgments
The authors would like to thank Dr Daniel Umbricht and Dr Solís-Vivanco for providing the means and SDs for their first-episode and control participants, and Dr Sherlyn Yeap, Dr John Foxe, Dr Ulrich Schall, and Dr Robert Oades for kindly providing additional information on their studies.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by National Institutes of Health RO1 grant (MH094328) to DFS.
Footnotes
Author Contributions
S. M. Haigh contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. B. A. Coffman contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. D. F. Salisbury contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kasai K, Shenton M, Salisbury D, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. http://dx.doi.org/10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velakoulis D, Wood SJ, McGorry PD, Pantelis C. Evidence for progression of brain structural abnormalities in schizophrenia: beyond the neurodevelopmental model. Aust N Z J Psychiatry. 2000;34(suppl 2):S113–S126. doi: 10.1080/000486700231. doi:10.1080/000486700231. [DOI] [PubMed] [Google Scholar]
- 3.Ho B, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. http://dx.doi.org/10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 4.DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res Neuroimaging. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. doi:10.1016/S0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 5.Lam M, Collinson SL, Sim K, Mackay CE, James AC, Crow TJ. Asymmetry of lexico-semantic processing in schizophrenia changes with disease progression. Schizophr Res. 2015;134:125–130. doi: 10.1016/j.schres.2011.10.020. doi:10.1016/j.schres.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Qiu L, Yuan L, et al. Evidence for progressive brain abnormalities in early schizophrenia: a cross-sectional structural and functional connectivity study. Schizophr Res. 2015;159:31–35. doi: 10.1016/j.schres.2014.07.050. doi:10.1016/j.schres.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. doi:10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. doi:10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 9.Srihari VH, Shah J, Keshavan MS. Is early intervention for psychosis feasible and effective? Psychiatr Clin North Am. 2012;35:613–631. doi: 10.1016/j.psc.2012.06.004. doi:10.1016/j.psc.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eack SM, Greenwald DP, Hogarty SS, Keshavan MS. One-year durability of the effects of cognitive enhancement therapy on functional outcome in early schizophrenia. Schizophr Res. 2010;120:210–216. doi: 10.1016/j.schres.2010.03.042. doi:10.1016/j.schres.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegelstad W, ten V, Larsen TK, Auestad B, et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am J Psychiatry. 2012;169:374–380. doi: 10.1176/appi.ajp.2011.11030459. doi:10.1176/appi.ajp.2011.11030459. [DOI] [PubMed] [Google Scholar]
- 12.Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1:182–188. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Näätänen R. The mismatch negativity: a powerful tool for cognitive neuroscience. Ear Hear. 1995;16:6–18. [PubMed] [Google Scholar]
- 14.Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16:38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Scherg M, Vajsar J, Picton T. A source analysis of the late human auditory evoked potentials. Cogn Neurosci J. 1989;1:336–355. doi: 10.1162/jocn.1989.1.4.336. doi:10.1162/jocn.1989.1.4.336. [DOI] [PubMed] [Google Scholar]
- 16.Hari R, Hämäläinen M, Ilmoniemi R, et al. Responses of the primary auditory cortex to pitch changes in a sequence of tone pips: neuromagnetic recordings in man. Neurosci Lett. 1984;50:127–132. doi: 10.1016/0304-3940(84)90474-9. doi:10.1016/0304-3940(84)90474-9. [DOI] [PubMed] [Google Scholar]
- 17.Gomot M, Giard M-H, Roux S, Barthélémy C, Bruneau N. Maturation of frontal and temporal components of mismatch negativity (MMN) in children. Neuroreport. 2000;11:3109–3112. doi: 10.1097/00001756-200009280-00014. [DOI] [PubMed] [Google Scholar]
- 18.Näätänen R, Sussman ES, Salisbury D, Shafer VL. Mismatch negativity (MMN) as an index of cognitive dysfunction. Brain Topogr. 2014;27:451–466. doi: 10.1007/s10548-014-0374-6. doi:10.1007/s10548-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Näätänen R, Shiga T, Asano S, Yabe H. Mismatch negativity (MMN) deficiency: a break-through biomarker in predicting psychosis onset. Int J Psychophysiol. 2015;95:338–344. doi: 10.1016/j.ijpsycho.2014.12.012. doi:10.1016/j.ijpsycho.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. doi:10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Baldeweg T, Klugman A, Gruzelier J, Hirsch SR. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr Res. 2004;69:203–217. doi: 10.1016/j.schres.2003.09.009. doi:10.1016/j.schres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19:1624–1632. doi: 10.1162/jocn.2007.19.10.1624. doi:10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-d -aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreitschmann-Andermahr I, Rosburg T, Demme U, Gaser E, Nowak H, Sauer H. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Cogn Brain Res. 2001;12:109–116. doi: 10.1016/s0926-6410(01)00043-x. doi:10.1016/S0926-6410(01)00043-X. [DOI] [PubMed] [Google Scholar]
- 25.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. doi:10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 26.Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51:400–406. doi: 10.1016/s0006-3223(01)01242-2. doi:10.1016/S0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- 27.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. doi:10.1016/S0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 28.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2004;10:40–68. doi: 10.1038/sj.mp.4001558. doi:10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 29.Akbarian S, Sucher NJ, Bradley D, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Errico F, Napolitano F, Squillace M, et al. Decreased levels of d -aspartate and NMDA in the prefrontal cortex and striatum of patients with schizophrenia. J Psychiatr Res. 2013;47:1432–1437. doi: 10.1016/j.jpsychires.2013.06.013. doi:10.1016/j.jpsychires.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Umbricht D, Koller R, Schmid L, et al. How specific are deficits in mismatch negativity generation to schizophrenia? Biol Psychiatry. 2003;53:1120–1131. doi: 10.1016/s0006-3223(02)01642-6. doi:10.1016/S0006-3223(02)01642-6. [DOI] [PubMed] [Google Scholar]
- 32.Baldeweg T, Hirsch SR. Mismatch negativity indexes illness-specific impairments of cortical plasticity in schizophrenia: a comparison with bipolar disorder and Alzheimer’s disease. Int J Psychophysiol. 2015;95:145–155. doi: 10.1016/j.ijpsycho.2014.03.008. doi:10.1016/j.ijpsycho.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Nagai T, Tada M, Kirihara K, Araki T, Jinde S, Kasai K. Mismatch negativity as a “translatable” brain marker toward early intervention for psychosis: a review. Front Psychiatry. 2013;4:115. doi: 10.3389/fpsyt.2013.00115. doi:10.3389/fpsyt.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumiyoshi T, Miyanishi T, Seo T, Higuchi Y. Electrophysiological and neuropsychological predictors of conversion to schizophrenia in at-risk subjects. Front Behav Neurosci. 2013;7:148. doi: 10.3389/fnbeh.2013.00148. doi:10.3389/fnbeh.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brockhaus-Dumke A, Tendolkar I, Pukrop R, Schultze-Lutter F, Klosterkötter J, Ruhrmann S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr Res. 2005;73:297–310. doi: 10.1016/j.schres.2004.05.016. doi:10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Shin KS, Kim JS, Kang D-H, et al. Pre-attentive auditory processing in ultra-high-risk for schizophrenia with magnetoencephalography. Biol Psychiatry. 2009;65:1071–1078. doi: 10.1016/j.biopsych.2008.12.024. doi:10.1016/j.biopsych.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Niznikiewicz MA, Spencer KM, Dickey C, et al. Abnormal pitch mismatch negativity in individuals with schizotypal personality disorder. Schizophr Res. 2009;110:188–193. doi: 10.1016/j.schres.2008.10.017. doi:10.1016/j.schres.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.del Re EC, Spencer KM, Oribe N, et al. Clinical high risk and first episode schizophrenia: auditory event-related potentials. Psychiatry Res Neuroimaging. 2015;231:126–133. doi: 10.1016/j.pscychresns.2014.11.012. doi:10.1016/j.pscychresns.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grzella I, Müller BW, Oades RD, et al. Novelty-elicited mismatch negativity in patients with schizophrenia on admission and discharge. J Psychiatry Neurosci. 2001;26:235–246. [PMC free article] [PubMed] [Google Scholar]
- 40.Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Näätänen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. doi:10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Rudolph ED, Ells EM, Campbell DJ, et al. Finding the missing-stimulus mismatch negativity (MMN) in early psychosis: altered MMN to violations of an auditory gestalt. Schizophr Res. 2015;166:158–163. doi: 10.1016/j.schres.2015.05.028. doi:10.1016/j.schres.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur M, Battisti RA, Lagopoulos J, Ward PB, Hickie IB, Hermens DF. Neurophysiological biomarkers support bipolar-spectrum disorders within psychosis cluster. J Psychiatry Neurosci. 2012;37:313–321. doi: 10.1503/jpn.110081. doi:10.1503/jpn.110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higuchi Y, Sumiyoshi T, Seo T, Miyanishi T, Kawasaki Y, Suzuki M. Mismatch negativity and cognitive performance for the prediction of psychosis in subjects with at-risk mental state. PLoS One. 2013;8:e54080. doi: 10.1371/journal.pone.0054080. doi:10.1371/journal.pone.0054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higuchi Y, Seo T, Miyanishi T, Kawasaki Y, Suzuki M, Sumiyoshi T. Mismatch negativity and P3a/reorienting complex in subjects with schizophrenia or at-risk mental state. Front Behav Neurosci. 2014;8:172. doi: 10.3389/fnbeh.2014.00172. doi:10.3389/fnbeh.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur M, Lagopoulos J, Lee RSC, et al. Longitudinal associations between mismatch negativity and disability in early schizophrenia- and affective-spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:161–169. doi: 10.1016/j.pnpbp.2013.07.002. doi:10.1016/j.pnpbp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012;71:98–104. doi: 10.1016/j.biopsych.2011.08.023. doi:10.1016/j.biopsych.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:822–829. doi: 10.1016/j.pnpbp.2010.03.019. doi:10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Oknina LB, Wild-Wall N, Oades RD, et al. Frontal and temporal sources of mismatch negativity in healthy controls, patients at onset of schizophrenia in adolescence and others at 15 years after onset. Schizophr Res. 2005;76:25–41. doi: 10.1016/j.schres.2004.10.003. doi:10.1016/j.schres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Solís-Vivanco R, Mondragón-Maya A, León-Ortiz P, Rodríguez-Agudelo Y, Cadenhead KS, de la Fuente-Sandoval C. Mismatch negativity reduction in the left cortical regions in first-episode psychosis and in individuals at ultra high-risk for psychosis. Schizophr Res. 2014;158:58–63. doi: 10.1016/j.schres.2014.07.009. doi:10.1016/j.schres.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Mondragón-Maya A, Solís-Vivanco R, León-Ortiz P, et al. Reduced P3a amplitudes in antipsychotic naïve first-episode psychosis patients and individuals at clinical high-risk for psychosis. J Psychiatr Res. 2013;47:755–761. doi: 10.1016/j.jpsychires.2012.12.017. doi:10.1016/j.jpsychires.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 51.Nagai T, Tada M, Kirihara K, et al. Auditory mismatch negativity and P3a in response to duration and frequency changes in the early stages of psychosis. Schizophr Res. 2013;150:547–554. doi: 10.1016/j.schres.2013.08.005. doi:10.1016/j.schres.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Hsieh MH, Shan J-C, Huang W-L, et al. Auditory event-related potential of subjects with suspected pre-psychotic state and first-episode psychosis. Schizophr Res. 2012;140:243–249. doi: 10.1016/j.schres.2012.06.021. doi:10.1016/j.schres.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 53.Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, Hermens DF. MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophr Res. 2011;130:203–209. doi: 10.1016/j.schres.2011.03.025. doi:10.1016/j.schres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 54.Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–966. doi: 10.1016/j.biopsych.2010.09.057. doi:10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 55.Devrim-Üçok M, Keskin-Ergen HY, Üçok A. Mismatch negativity at acute and post-acute phases of first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258:179–185. doi: 10.1007/s00406-007-0772-9. doi:10.1007/s00406-007-0772-9. [DOI] [PubMed] [Google Scholar]
- 56.Magno E, Yeap S, Thakore JH, Garavan H, De Sanctis P, Foxe JJ. Are auditory-evoked frequency and duration mismatch negativity deficits endophenotypic for schizophrenia? High-density electrical mapping in clinically unaffected first-degree relatives and first-episode and chronic schizophrenia. Biol Psychiatry. 2008;64:385–391. doi: 10.1016/j.biopsych.2008.03.019. doi:10.1016/j.biopsych.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59:762–772. doi: 10.1016/j.biopsych.2005.08.030. doi:10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 58.Oades R, Wild-Wall N, Juran S, Sachsse J, Oknina L, Ropcke B. Auditory change detection in schizophrenia: sources of activity, related neuropsychological function and symptoms in patients with a first episode in adolescence, and patients 14 years after an adolescent illness-onset. BMC Psychiatry. 2006;6:7. doi: 10.1186/1471-244X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valkonen-Korhonen M, Purhonen M, Tarkka IM, et al. Altered auditory processing in acutely psychotic never-medicated first-episode patients. Cogn Brain Res. 2003;17:747–758. doi: 10.1016/s0926-6410(03)00199-x. doi:10.1016/S0926-6410(03)00199-X. [DOI] [PubMed] [Google Scholar]
- 60.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. doi:10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 61.Salisbury DF, Olizzotto NR, Haigh SM, Koehler J, McCarley RW. Pitch and duration MMN are not reduced in high functioning first hospitalized schizophrenia. [Google Scholar]
- 62.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum; Mahwah, NJ: 1988. [Google Scholar]
- 63.Hedges LV, Olkin I. Statistical Models for Meta-Analysis. Academic Press; Orlando, FL: 1985. [Google Scholar]
- 64.Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52:749–758. doi: 10.1016/s0006-3223(02)01379-3. doi:10.1016/S0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- 65.Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC. Differential relationships of mismatch negativity and visual P1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol Psychiatry. 2012;71:521–529. doi: 10.1016/j.biopsych.2011.10.037. doi:10.1016/j.biopsych.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall M-H, Schulze K, Rijsdijk F, et al. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley bipolar twin and family study. Psychol Med. 2009;39:1277–1287. doi: 10.1017/S0033291709005261. doi:10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- 67.Korostenskaja M, Dapsys K, Siurkute A, Maciulis V, Ruksenas O, Kähkönen S. Effects of olanzapine on auditory P300 and mismatch negativity (MMN) in schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:543–548. doi: 10.1016/j.pnpbp.2005.01.019. doi:10.1016/j.pnpbp.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 68.Umbricht D, Javitt D, Novak G, et al. Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol. 1999;2:299–304. doi: 10.1017/S1461145799001595. doi:10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- 69.Umbricht D, Javitt D, Novak G, et al. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998;44:716–725. doi: 10.1016/s0006-3223(97)00524-6. doi:10.1016/S0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 70.Horton J, Millar A, Labelle A, Knott VJ. MMN responsivity to manipulations of frequency and duration deviants in chronic, clozapine-treated schizophrenia patients. Schizophr Res. 2011;126:202–211. doi: 10.1016/j.schres.2010.11.028. doi:10.1016/j.schres.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Z, Zhu H, Chen L. Effect of aripiprazole on mismatch negativity (MMN) in schizophrenia. PLoS One. 2013;8:e52186. doi: 10.1371/journal.pone.0052186. doi:10.1371/journal.pone.0052186. [DOI] [PMC free article] [PubMed] [Google Scholar]