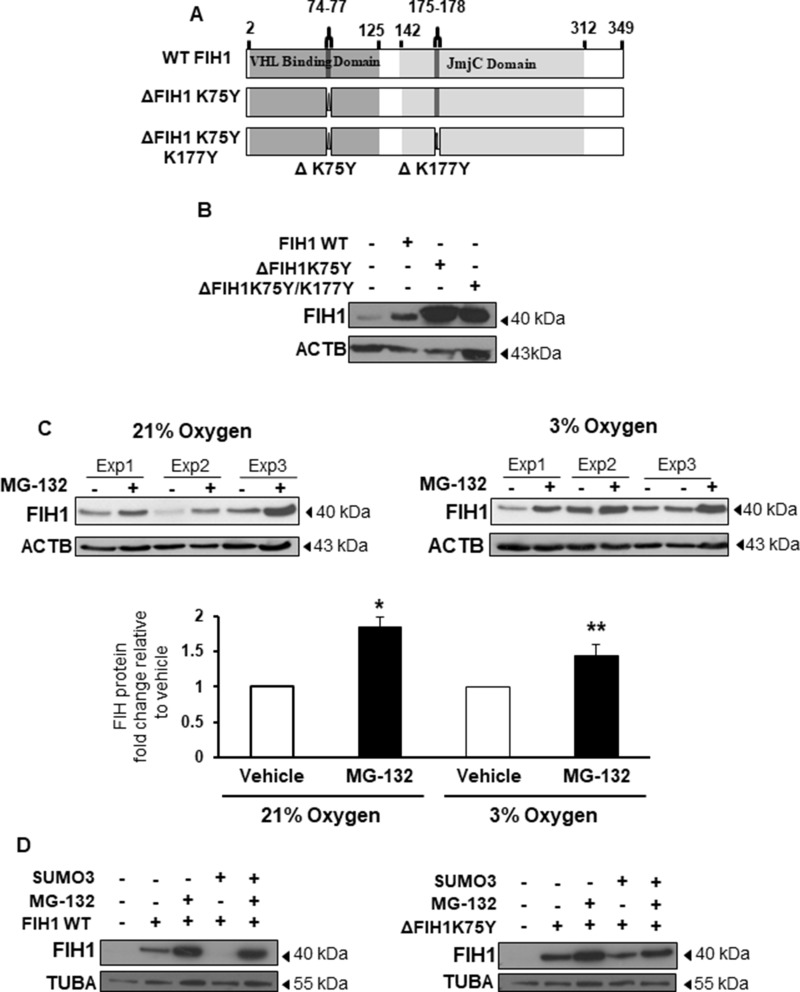
Figure 3. SUMOylation of FIH1 targets it for proteasomal degradation.
(A) Schematic representation of FIH1 protein wild type (WT) construct containing a VHL Binding Domain (dark grey), a JmjC domain (light grey), high probability (ΔK75Y) and low probability (ΔK177Y) SUMOylation sites. Mutated FIH1 constructs (ΔK75Y and ΔK75YΔK177Y respectively) were generated by single-point mutations. (B) Representative Western Blots of FIH1 following FIH1 overexpression using plasmids constructs containing either FIH1 WT, FIH1 ΔK75Y, or FIH1 ΔK75Y ΔK177Y. (C) Top panel: representative Western Blot of FIH1 in JEG-3 cells following treatment with the proteasome inhibitor (MG-132) in 21% (left) or 3% oxygen (right). Bottom panel: Densitometric analysis of FIH1 protein expression in MG-132 treated JEG-3 cells. Data are expressed as a fold change relative to control (V) vehicle (21% oxygen: n = 5, *p < 0.05, Mann-Whitney Test; 3% oxygen n = 5, **p < 0.01, Mann-Whitney Test). (D) Representative Western Blots for FIH1 in JEG-3 following overexpression of FIH1 WT (left panel) or FIH1 ΔK75Y (right panel) and SUMO3 protein in the presence and absence of the MG-132.