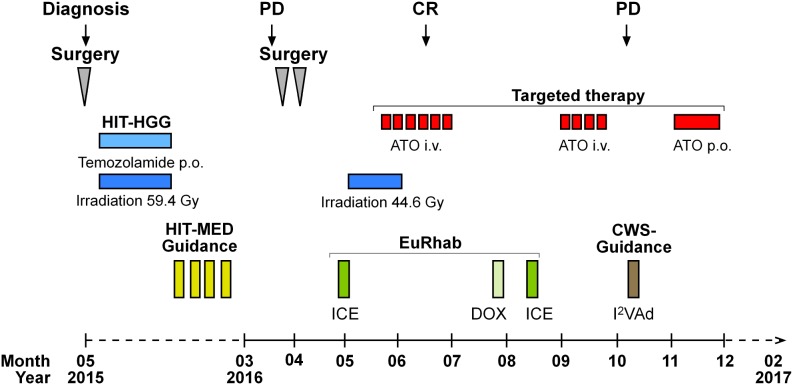
Figure 3. Therapy of P1.
With a presumed diagnosis of a malignant glioma, we initiated treatment according to the HIT-HGG protocol (cranial irradiation with 59.4 Gy in 30 fractions with concomitant oral temozolamide chemotherapy). Due to the following diagnosis of a “primitive neuroectodermal tumor with WNT-like subtype” we added 4 cycles of chemotherapy with vincristine, cisplatin and CCNU according to the HIT-Med protocol. The relapse treatment protocol combined conventional radio-chemotherapy with an individualized therapy approach. The back-bone chemotherapy, included elements from pediatric rhabdoid and soft-tissue sarcoma protocol (EURHAB; CWS-Register “SoTiSaR”, Soft Tissue Sarcoma Registry). After surgical resection, the sites of the metastatic skull lesions were irradiated with 44 Gy (5 x 2 Gy/week). ATO was administered to coincide with the last two weeks of radiation. We conducted two cycles of intravenous (i.v.) ATO, given five days per week for six weeks in the first cycle (ATO I) and for four weeks in the second cycle (ATO II) with an interval of eight weeks in between. Following the diagnosis of progressive, systemic disease, we switched to an oral (p.o.) ATO formulation for four weeks. PD and CR were assessed by MRI. PD = progressive disease; CR = clinical remission; ATO = arsenic trioxide; ICE = ifosfamide, carboplatin, etoposide; DOX = doxorubicin; I2VAd = ifosfamide, vincristine, dactinomycin C.