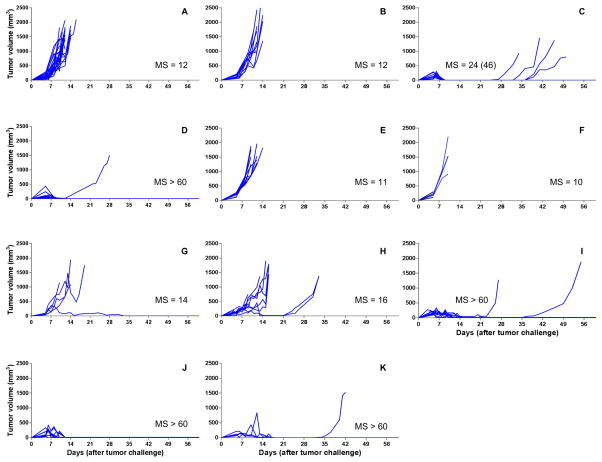
Figure 3. Spider plots for tumor volumes of EL4 challenged C57BL/6J mice receiving different 5-FU formulations.
Mice received the following treatments (on Day 5 post tumor challenge, when palpable tumor was present): A: naive mice without treatment (n = 24); B: mice treated with blank PLGA pellets (n = 10); C: mice treated with 5-FU solution by SC injection (120 mg/kg) (n = 10); D: mice treated with 5-FU solution by IP injection (120 mg/kg) (n = 10); E: mice treated with 5-FU-loaded pellets (30% w/w of 5-FU, 80 mg/kg) (n = 10); F: mice treated with 5-FU-loaded pellets (30% w/w 5-FU, 120 mg/kg) (n = 3); G: mice treated with 5-FU-loaded pellets (40% w/w 5-FU, 120 mg/kg) (n = 5); H: mice treated with 5-FU-loaded pellets (50% w/w 5-FU, 40 mg/kg) (n = 9); I: mice treated with 5-FU-loaded pellets (50% w/w 5-FU, 80 mg/kg) (n = 10); J: mice treated with 5-FU-loaded pellets (50% w/w 5-FU, 120 mg/kg) (n = 10); K: mice treated with 5-FU-loaded pellets (40% w/w 5-FU, 120 mg/kg) combined with 5-FU-loaded pellets (50% w/w 5-FU, 40 mg/kg) (n = 4). Median survival times (MS, days) using all-cause mortality are shown besides the spider plots of each group. The median survival times when events were death from no efficacy are equal to those when all-cause mortality was used, unless otherwise indicated in parentheses.