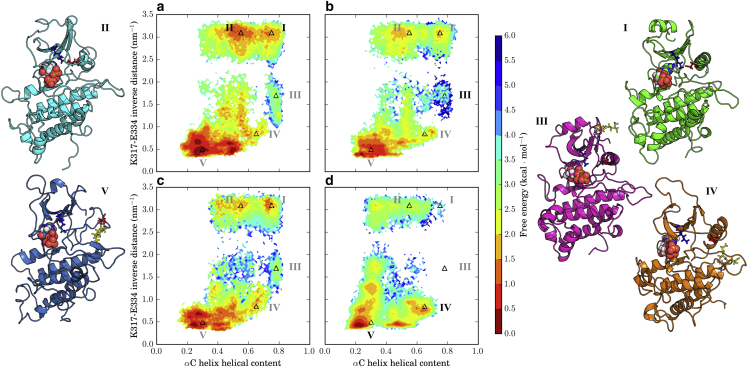
Figure 4.
Effects of S-glutathionylation on prevalence of the active-like state in BAK1. MSM-weighted free-energy plots projected onto the αC helix helical content and the inverse K317-E334 distance from simulations of (a) nonglutathionylated BAK1, and BAK1 S-glutathionylated on (b) C353, (c) C374, and (d) C408. Points on the plane with representative structures shown are demarcated by triangles labeled in black, and correspond to the following regions: I) BAK1-SH, active-like state; II) BAK1-SH, formed K-E salt bridge and unfolded αC helix; III) BAK1-C353SG, slightly broken K-E salt bridge and formed αC helix; IV) BAK1-C408SG, broken K-E salt bridge and partially unfolded αC helix; and V) BAK1-C408SG, broken K-E salt bridge and unfolded αC helix. To see this figure in color, go online.