Figure 2.
MALDI-TOF Analysis of the AAV5 VP1, VP2, and VP3 Capsid Proteins Stoichiometry
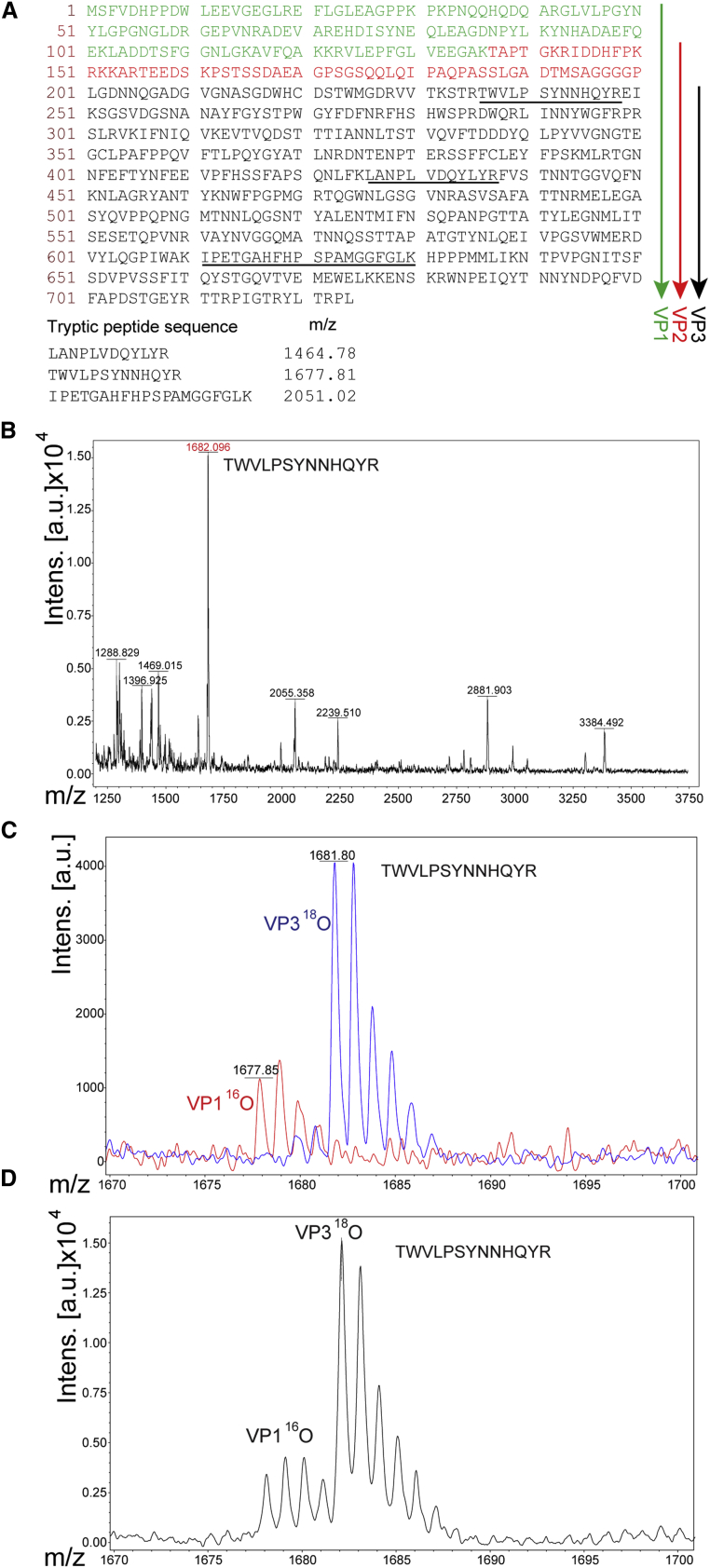
(A) Amino acid sequence of the AAV5 capsid with VP1 unique N termini marked in green, unique VP2 in red, and common VP3 C termini in black. The downward arrows indicate the respective proteins. Tryptic peptides selected for MS analysis are underlined and shown below their respective observed masses. (B) MALDI-TOF-MS spectrum of all tryptic peptides of rAAV5 digested in H218O. The red highlighted peptide is one representative out of three analyzed. (C) Two overlaid MALDI-TOF MS spectra of the same tryptic peptide TWVLPSYNNHQYR originating from the VP1 gel band digested with trypsin prepared in 16O water (red trace) or from the VP3 gel band digested with trypsin prepared in 18O water (blue trace). 18O water incorporates two 18O atoms on the C terminus of the peptide, thus shifting the mass by 4 atomic mass units (amu). These digestion products were spotted/analyzed separately and the spectra overlaid to show the complete incorporation of two 18O into the VP3 peptide. (D) Isotopic “fingers” of the same peptide derived from VP1 or VP3 after the digestion products were mixed at 1:1 ratios to calculate the relative content. See also Tables S2 and S3 and Figure S3.