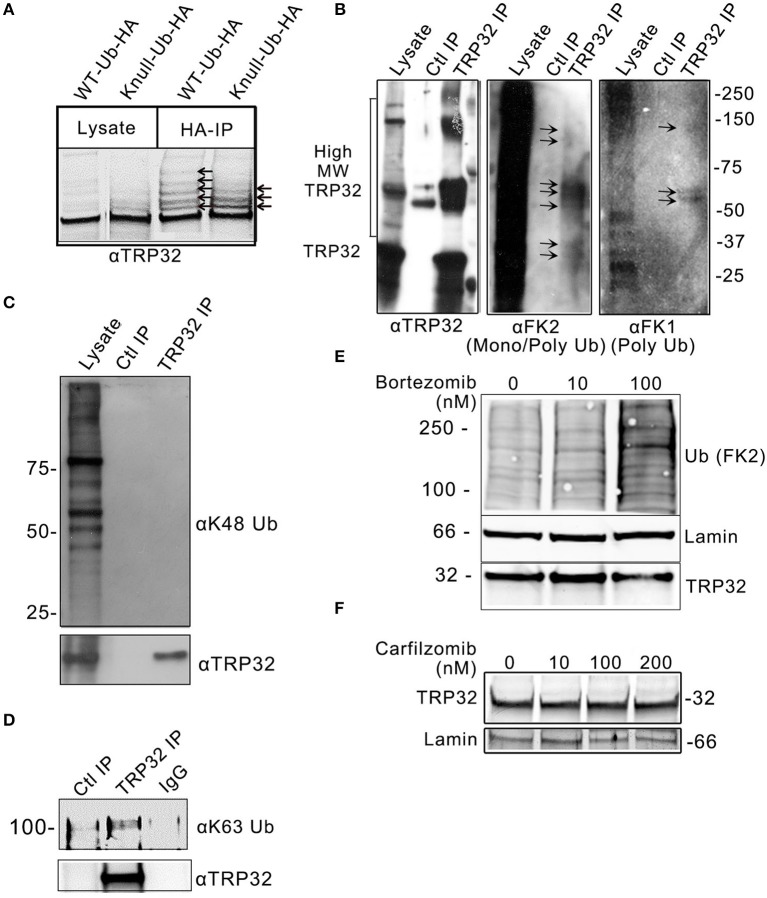
Figure 2.
E. chaffeensis TRP32 is mono and polyubiquitinated, but is not degraded by the proteasome. (A) GFP-tagged TRP32 was cotransfected into HeLa cells with HA-tagged WT and K-null Ub constructs. HA-immunoprecipitation was performed and the resulting eluate was probed with anti-TRP32 specific antibody by immunoblot. Multiple higher molecular weight bands indicative of ubiquitinated species of TRP32 were detected (arrows). (B) Multiple species of ubiquitinated TRP32 were also detected during infection of THP-1 cells. Anti-TRP32 specific antibody was used to immunoprecipitate TRP32 from infected THP-1 lysate. The resultant immunoprecipitated protein was probed with antibodies specific for conjugated mono and polyUb chains (FK2) and for polyUb chains alone (FK1) by immunoblot. Bands were detected (arrows) between 25 and 37 kDa, 50 and 75 kDa, and above 100 kDa that colocalized with TRP32 bands. Immunoprecipitation of TRP32 and immunoblot with anti-K48 and anti-K63 Ub antibodies show absence of K48 ubiquitination (C) and presence of K63-linked polyubiquitinated TRP32 (D). TRP32 levels were unaffected by treatment with a proteasome inhibitor. E. chaffeensis-infected cells were incubated with varying concentrations of the proteasome inhibitor bortezomib (E) for 12 h or carfilzomib (F) for 8 h prior to harvest and immunoblotting with anti-TRP32 and anti-lamin (ctrl) and anti-Ub (FK2) antibodies.