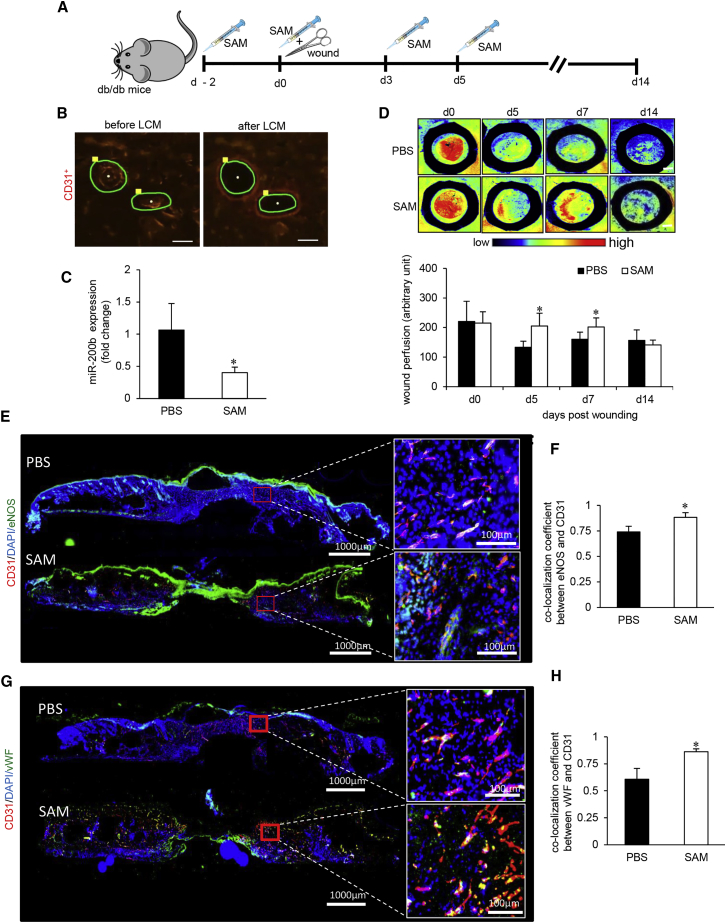
Figure 4.
SAM Reversed Diabetes-Associated Impairment in Wound Vascularization
(A) Schematic diagram showing intradermal delivery of S-adenosylmethionine (SAM) or vehicle to the dorsal skin of db/db mice. Two 8-mm diameter full thickness stent wounds were created on dorsal skin on day 0. (B) Representative figure shows the selection of CD31+ tissue elements (red), and their collection before and after the LCM from the wound edge tissues. Scale bar, 150 μm. (C) qRT-PCR analysis of miR-200b levels in endothelial elements of SAM-treated diabetic wounds compared to placebo. n = 3, *p < 0.05 (Student’s t test). (D) Perimed Laser speckle-assisted wound perfusion analysis of diabetic wounds administered with SAM or placebo. n = 5, *p < 0.05 (Student’s t test). (E and F) Immunohistochemical analysis of eNOS+/CD31+ co-expression (E) and its co-localization analysis (F) in SAM- or PBS-administered diabetic wounds. (G and H) Immunohistochemical analysis of vWF+/CD31+ co-expression (G) and its co-localization analysis (H) in SAM- or PBS-administered diabetic wounds. n = 5, *p < 0.05 (Student’s t test). Data represented as the mean ± SD.