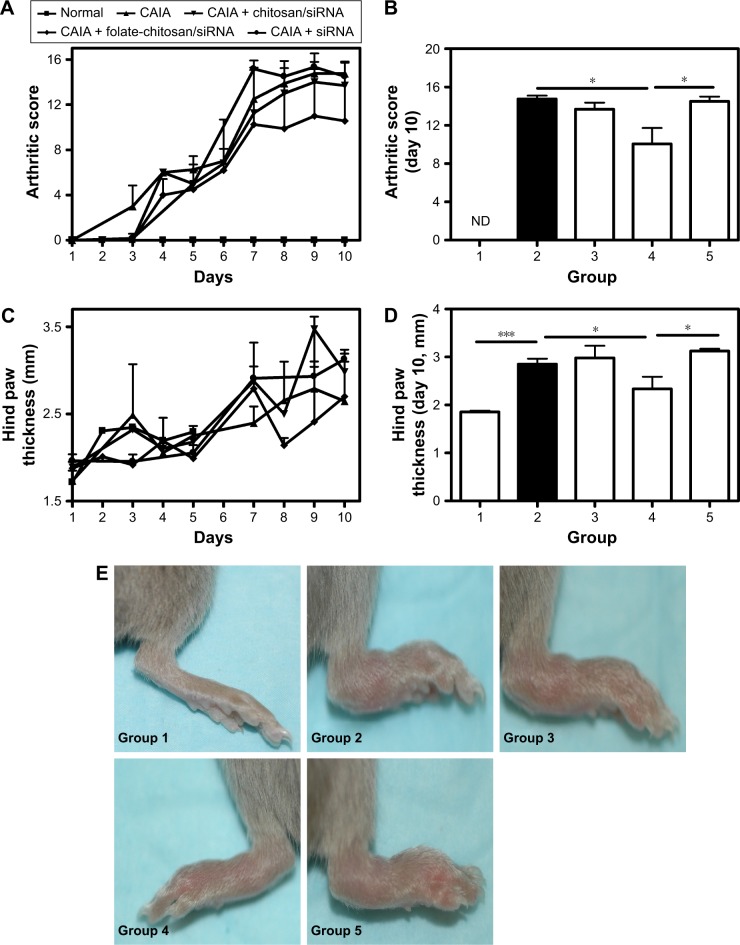
Figure 2.
Arthritis progression and the therapeutic effects of CH-DEAE15/siRNA-TNFα, folate-PEG-CH-DEAE15/siRNA-TNFα and naked siRNA-TNFα nanoparticles in a murine CAIA model.
Notes: (A) Joint inflammation in mice was measured by an arthritic scoring method22 to verify disease progression on a scale of 0–4 for each paw, for a total score of 0–16 for all four paws. On the first day (day 1), arthritis was induced by ip injection of a 1.5 mg cocktail of arthritogenic mAb against type II collagen. Two days later (day 3), mice received an ip injection with 50 µg Escherichia coli (0.5 mg/mL stock) lipopolysaccharide. At the same time on days 1, 3, 5 and 7, mice received an ip injection with 100 µL of nanoparticles containing the equivalent of 50 µg siRNA-TNFα. (B) Arthritic score on day 10. (C) Arthritis development estimated by measuring hind paw thickness over the course of the experiment. (D) Hind paw thickness on day 10. (E) Hind paws of mice on day 10. Statistical significance was assessed by unpaired Student’s t-test, *P<0.05, ***P<0.001. Each group contained eight mice except group 5 which only had five mice. Group 1: normal control; group 2: CAIA control; group 3: CAIA mice treated with CH-DEAE15/siRNA-TNFα nanoparticles; group 4: CAIA mice treated with folate-PEG-CH-DEAE15/siRNA-TNFα nanoparticles; group 5: CAIA mice treated with siRNA-TNFα.
Abbreviations: CAIA, collagen antibody-induced arthritis; CH, chitosan; DEAE, diethylethylamine; ip, intraperitoneal; mAb, monoclonal antibody; ND, not determined; PEG, polyethylene glycol; SEM, standard error of the means; TNFα, tumor necrosis factor-alpha.