ABSTRACT
All vertebrate embryos are exposed to maternally derived steroids during development. In placental vertebrates, metabolism of maternal steroids by the placenta modulates embryonic exposure, but how exposure is regulated in oviparous vertebrates is less clear. Recent work in oviparous vertebrates has demonstrated that steroids are not static molecules, as they can be converted to more polar steroid sulfates by sulfotransferase enzymes. Importantly, these steroid sulfates can be converted back to the parent compound by the enzyme steroid sulfatase (STS). We investigated when and where STS was present during embryonic development in the red-eared slider turtle, Trachemys scripta. We report that STS is present during all stages of development and in all tissues we examined. We conclude that STS activity may be particularly important for regulating maternal steroid exposure in oviparous vertebrates.
KEY WORDS: Steroid metabolism, Oviparous vertebrate, Maternal effects, Reptile
Summary: Steroid sulfatase activity hydrolyzes steroid sulfates back to an active form. Here we demonstrate steroid sulfatase is present in various tissues during embryonic development.
INTRODUCTION
Steroids are important mediators of embryonic development as they are capable of influencing phenotypes in all vertebrates (Cooke et al., 1998). The developmental effects of steroids are often studied in relation to sexual differentiation, where sex steroids produced by embryonic gonads play a role in producing irreversible, sex-specific effects ranging from differentiation of reproductive organs to sex-specific reproductive behaviors in adults (Goy and McEwen, 1980; Ball et al., 2014). Other steroids also affect embryonic development as evidenced by the vital role glucocorticoids play in preparing developing lungs and other tissues for conditions outside of the maternal environment (Ballard, 1979; Fowden et al., 1998; Moisiadis and Matthews, 2014). These studies demonstrate that steroids produced by the embryo are important modulators of offspring phenotype, but they are not the only steroids present during development.
All vertebrate embryos develop in the presence of maternally derived steroids. In oviparous vertebrates, steroids of maternal origin are present in eggs at the time of laying (Schwabl, 1993), and these yolk steroids have the potential to affect offspring phenotype (Williams and Groothuis, 2015). Yolk steroids can influence various aspects of offspring phenotype such as behavior, growth and immune function (Groothuis et al., 2005). In birds, elevated yolk testosterone can influence traits early in life such as nestling begging behavior (von Engelhardt et al., 2006), as well as traits later in life such as mate choice in adults (Hsu et al., 2016). This research has led to the hypothesis that yolk steroids might have important fitness consequences for both mother and offspring (reviewed in Groothuis et al., 2005). While there are many examples of maternal steroids influencing offspring traits (Williams and Groothuis, 2015), our understanding of the physiological processes underlying these effects lags far behind (reviewed in Moore and Johnston, 2008; Groothuis and Schwabl, 2008). Basic questions such as ‘how does exposure to maternal hormones affect individual phenotype without interfering with sexual differentiation?’ (Carere and Balthazart, 2007; Moore and Johnston, 2008) are, at best, only beginning to be addressed, highlighting the need for studies that examine the mechanisms underlying yolk steroid effects (Williams, 2012; Williams and Groothuis, 2015).
There is an increasing focus on deciphering the physiological processes underlying the effects of yolk steroids, with an emphasis on understanding the metabolic fate of maternal steroids. Findings from a variety of oviparous species and several different steroids demonstrate that maternal steroids are metabolized during early embryonic development, which modulates embryonic exposure to these steroids (reviewed in Paitz and Bowden, 2013). These studies have primarily relied on injecting radiolabeled steroids into either the yolk or albumen of eggs and have demonstrated that the in ovo metabolism of androgens (von Engelhardt et al., 2009; Paitz et al., 2011; Benowitz-Fredericks and Hodge, 2013; Paitz and Bowden, 2013), progesterone (Paitz and Casto, 2012; Paitz and Bowden, 2013), corticosterone (von Engelhardt et al., 2009; Vassallo et al., 2014) and estrogens (Paitz et al., 2012; Paitz and Bowden, 2015) often occurs within the first 5 days of embryonic development, resulting in the production of metabolites that are much more polar and water soluble. One such study using these methods demonstrated that the primary metabolite formed in eggs of the red-eared slider turtle, Trachemys scripta (Schoepff 1792), is estrone sulfate (Paitz and Bowden, 2013). Given that in ovo metabolism results in steroids being converted to more polar forms, some of which have been identified as sulfonated forms, it is possible that maternal steroids are sulfonated in ovo. Interestingly, the sulfonation of maternal steroids is also the primary pathway of steroid metabolism in placental vertebrates, including humans (Diczfalusy, 1969), indicating that this pathway is likely conserved across vertebrates and results in embryonic exposure to steroid sulfates (Paitz and Bowden, 2013).
In addition to steroid sulfates being produced from the metabolism of yolk steroids during early development, there are detectable levels of maternally derived steroid sulfates present in eggs (Haccard et al., 2012; Paitz and Bowden, 2013). At present, little is known about the biological effects of steroid sulfates, but a common premise is that these metabolites represent inactive end products as the sulfonation of a steroid typically renders it unable to bind its respective steroid receptor and results in an increased clearance rate via excretion (reviewed in Strott, 2002). However, there is evidence suggesting this may not always be the case. For example, both estradiol sulfate and estrone sulfate alter sex ratios in T. scripta when applied exogenously (Paitz and Bowden, 2011, 2013). As steroid sulfates do not bind steroid receptors, most effects attributed to steroid sulfates are thought to result from the desulfonation of the metabolite by the enzyme steroid sulfatase (STS), which is the sole enzyme responsible for the hydrolysis of steroid sulfates back to their active form (Reed et al., 2005). In humans, STS is most abundant in the placenta (Miki et al., 2002), where it converts steroid sulfates produced by the fetus back to free steroids that enter maternal circulation (Goodman, 2003). The loss of STS activity results in elevated levels of all steroid sulfates (Reed et al., 2005), indicating the importance of this enzyme in the conversion of steroid metabolites. We know that STS is abundant during embryonic development in placental vertebrates, and it is able to produce biologically active steroids from steroid sulfates, but whether STS is present in oviparous vertebrates during development is not known.
In the present study, we investigated whether STS is present in embryos and extraembryonic membranes of T. scripta across development. In T. scripta, concentrations of maternally derived estradiol vary dramatically across the nesting season, with a female's second clutch containing approximately 10 times the amount of estradiol as her first clutch (Paitz and Bowden, 2009). Once incubation begins, concentrations of estradiol within the yolk decline rapidly (Paitz and Bowden, 2009) as a result of estradiol being metabolized to estrone sulfate (Paitz and Bowden, 2008), which accumulates within the yolk and reaches the embryo during the final third of development (Paitz et al., 2012). If STS is present during development, it would suggest that the sulfonated metabolites of maternal steroids could, generally, serve as precursors for the production of active steroids that could, in turn, influence offspring development.
MATERIALS AND METHODS
Egg collection and incubation
During June 2011, gravid females were collected from a marked population of T. scripta that inhabits Banner Marsh State Fish and Wildlife Area, and returned to the laboratory where eggs were obtained via oxytocin injection (Ewert and Legler, 1978; Les et al., 2009). Five clutches (n=61 eggs) were used for this study, with each clutch being evenly divided between two incubation treatments: as T. scripta possesses temperature-dependent sex determination (Crews et al., 1994), half of the eggs from each clutch were incubated at 26°C to produce male hatchlings and the other half were incubated at 31°C to produce female hatchlings. These temperatures are known to produce 100% males and 100% females, respectively (Crews et al., 1994). Because embryos develop at different rates under these two conditions, eggs in the cooler treatment were sampled on days 13, 26, 39, 53, 65 and 70 of development while eggs in the warmer treatment were sampled on days 8, 16, 24, 32, 40 and 48 of development, with five eggs sampled on each sampling day. This approach resulted in embryos being sampled at approximately equivalent developmental stages and evenly covering the entire incubation period for both temperatures (Greenbaum, 2002; Paitz and Bowden, 2009). At the time of sampling, extra-embryonic membranes, liver, brain and adrenal–kidney–gonad complexes (AKGs) were removed, except for early stage embryos (stages 14–16), which were too small for collection of individual tissues, so whole embryos were collected. All samples were frozen on dry ice, and stored at −20°C until enzyme assays were conducted. This work was approved the Illinois State University IACUC Committee.
STS quantification and analysis
STS activity was quantified by measuring the conversion of [3H]estrone sulfate (E1S) to [3H]estrone (E1) following Miki et al. (2002). First, tissues were homogenized in homogenization buffer (250 nmol l−1 sucrose, 5 mmol l−1 MgCl2, 100 mmol l−1 Tris-HCl pH 7.4) and centrifuged for 10 min at 1000 g. The protein concentration of the supernatant was determined via a Bradford assay and all supernatants were diluted to a final concentration of 3 µg µl−1. Next, 20 µl of supernatant (60 µg of protein) was added to 100 µl of assay buffer (0.2 mol l−1 sodium acetate) containing 50,000 cpm of [3H]estrone sulfate (specific activity 57.3 Ci mmol−1; Perkin Elmer, Waltham, MA, USA), 25 mmol l−1 sucrose and 4 mmol l−1 nicotinamide (Miki et al., 2002). Reactions were run for 2 h at 31°C and terminated with the addition of 1 ml of ice-cold toluene, and vortexed for 30 s. Newly hydrolyzed [3H]estrone was quantified by measuring radioactivity levels in 500 µl of toluene. Thin-layer chromatography was used to confirm that the toluene-soluble radioactivity migrated with an authentic estrone standard (Steraloids, Newport, RI, USA) and that [3H]estrone sulfate was not present in the toluene (Paitz et al., 2012). STS activity was calculated as estrone produced (cpm) µg−1 protein h−1.
To test for differences in STS activity, a mixed-model ANOVA was used with developmental stage, incubation temperature and tissue type (along with all interactions) included as fixed factors, while clutch identity was included as a random factor. Values were log transformed to normalize the data prior to analysis. Group means were compared using post hoc comparisons (Tukey's HSD).
RESULTS AND DISCUSSION
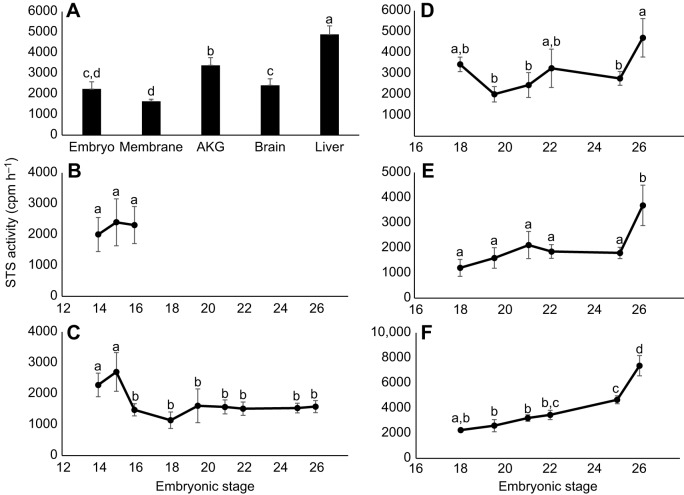
We found that STS activity was present in all tissues tested across development (Fig. 1, Table S1). Incubation temperature did not affect STS activity (F1,151=3.07, P=0.082), suggesting males and females exhibit similar patterns of STS activity. Levels of STS activity varied by tissue (F4,151=34.63, P<0.001), being highest in the liver, intermediate in the brain and AKGs, and lowest in extra-embryonic membranes and whole embryos (Fig. 1A). STS activity changed across development, but the pattern of change differed by tissue as evidenced by the significant interaction of tissue and developmental stage (F17,151=2.19, P=0.007). Levels in whole embryos and AKGs remained relatively stable across development (Fig. 1B,D). STS levels in the extra-embryonic membranes were highest early in development, declining later in development (Fig. 1C), while levels in the brain and liver were lowest during early development, increasing later in development (Fig. 1E,F).
Fig. 1.
Steroid sulfatase activity in Trachemysscripta embryos. (A) Mean (±s.e.m.) steroid sulfatase (STS) activity for each tissue averaged across development. (B–F) STS activity within the (B) embryo (n=17), (C) membranes (n=61), (D) adrenal–kidney–gonad complexes (AKGs; n=39), (E) brain (n=39) and (F) liver (n=39) across development. STS activity was calculated as estrone produced (cpm) µg−1 protein h−1. Groups not sharing a letter are significantly different (P<0.05) as measured by ANOVA. Non-transformed values are plotted.
Embryonic development is a period marked by high sensitivity to steroids (reviewed in Cooke et al., 1998) as well as high levels of steroid sulfates (Loriaux et al., 1972; Paitz and Bowden, 2013), and here we demonstrate that STS is present in all tissues examined. Given that STS is capable of converting most, if not all, steroid sulfates back to their active form (Mueller et al., 2015), the broad distribution of STS has implications for developing embryos. The prevalence of STS, paired with the prevalence of steroid sulfates, suggests that STS is an important, yet understudied, route of steroid signaling during development, and the interplay of STS and steroid sulfates could ultimately dictate when and where maternally derived steroids elicit their effects.
In T. scripta, estrone sulfate is present in eggs as a maternally derived compound (Paitz and Bowden, 2013), and is also produced from the metabolism of maternal estradiol (Paitz and Bowden, 2011; Paitz et al., 2012). Given that estrone sulfate is abundant in yolk and albumen at the onset of development and is taken up by the embryo throughout development (Paitz et al., 2012), it is possible that STS can affect embryonic development by converting estrone sulfate back to an active form to influence developmental processes such as sex determination (Paitz and Bowden, 2013). While most research in T. scripta has focused on estrone sulfate, numerous other steroid sulfates have also been shown to be present during development (Paitz and Bowden, 2013), but their fate is presently unknown. Ultimately, the importance of STS as a modulator of embryonic development will depend on the presence of steroid sulfates available for reactivation.
Steroid sulfates are abundant during pregnancy, and numerous studies have examined STS activity during embryogenesis in placental vertebrates. Only recently has it become apparent that steroid sulfates are also abundant during development in oviparous vertebrates (Haccard et al., 2012; Paitz and Bowden, 2013). Patterns of STS activity in embryos of placental vertebrates are relatively similar to what we report in T. scripta; in mice (Compagnone et al., 1997) and humans (Miki et al., 2002), STS activity is high during the later stages of development and subsequently drops after birth such that embryonic levels of STS activity are higher than adult levels (Mortaud et al., 1996; Miki et al., 2002). However, STS levels tend to be highest within the placenta itself (Miki et al., 2002), which is thought to facilitate the movement of steroids from the fetal circulation into the maternal circulation for subsequent excretion (Levitz et al., 1960; Reed et al., 2005), but we found the lowest levels in the extra-embryonic membranes. Given that there is no opportunity to transfer steroids back to the maternal environment in oviparous vertebrates, it is not surprising that we did not detect high STS activity in T. scripta extra-embryonic membranes.
Variation in substrate availability will dictate the consequences of STS activity; thus, it is important to understand the dynamics of steroid sulfate production during embryonic development. The hydrophilic nature of steroid sulfates means that active transport is necessary for both cellular uptake and efflux, and this is primarily carried out by solute carrier transporters and ATP-binding cassette transporters (Mueller et al., 2015). Functionally, this means that the movement of steroid sulfates can be more tightly controlled than the movement of free steroids, and that STS activity can alter the transport and movement of steroids by freeing them from this controlled transport. The ability to regulate the movement of molecules may be even more important for embryos of oviparous vertebrates as there is no opportunity to move unwanted molecules to the maternal circulation for excretion. Additionally, the finite amount of resources available in eggs for development could necessitate the recycling of molecules in a coordinated fashion. It is possible that STS interacts with the sulfotransferase enzymes that produce steroid sulfates to modulate the movement of maternal steroids throughout development, allowing molecules to be repeatedly conjugated and deconjugated. If correct, maternal steroid effects could arise in various tissues across development, and this could explain many of the pleiotropic effects that have been observed (Williams and Groothuis, 2015). A continued investigation into the prevalence of STS and steroid sulfates during embryonic development is necessary to better understand the role of this pathway in regulating development, including potential effects of maternal steroids.
Supplementary Material
Acknowledgements
We thank the IDNR for access to Banner Marsh and Pat Wager for assistance with sample collection.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: R.T.P., R.M.B.; Methodology: R.T.P., K.R.D.; Validation: R.T.P.; Investigation: K.R.D., R.M.B.; Resources: R.T.P., R.M.B.; Data curation: R.T.P.; Writing - original draft: R.T.P., R.M.B.; Writing - review & editing: R.T.P., K.R.D., R.M.B.; Supervision: R.T.P., R.M.B.; Funding acquisition: R.T.P., R.M.B.
Funding
This work was supported by National Science Foundation IOS-0,952,840 to R.M.B. and National Institutes of Health R15ES023995 to R.M.B. and R.T.P. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.167031.supplemental
References
- Ball G. F., Balthazart B. and McCarthy M. M. (2014). Is it useful to view the brain as a secondary sexual characteristic? Neurosci. Biobehav. Rev. 46, 628-638. 10.1016/j.neubiorev.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Ballard P. L. (1979). Glucocorticoids and differentiation. Monogr. Endocrinol. 12, 493-515. 10.1007/978-3-642-81265-1_26 [DOI] [PubMed] [Google Scholar]
- Benowitz-Fredericks Z. M. and Hodge M. (2013). Yolk androstenedione in domestic chicks (Gallus gallus domesticus): uptake and sex-dependent alteration of growth and behavior. Gen. Comp. Endocrinol. 193, 48-55. 10.1016/j.ygcen.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Carere C. and Balthazart J. (2007). Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends. Endocrinol. Metab. 18, 73-80. 10.1016/j.tem.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Compagnone N. A., Salido E., Shapiro L. J. and Mellon S. H. (1997). Expression of steroid sulfatase during embryogenesis. Endocrinology 138, 4768-4773. 10.1210/endo.138.11.5504 [DOI] [PubMed] [Google Scholar]
- Cooke B., Hegstrom C. D., Villeneuve L. S. and Breedlove S. M. (1998). Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 19, 323-362. 10.1006/frne.1998.0171 [DOI] [PubMed] [Google Scholar]
- Crews D., Bergeron J. M., Bull J. J., Flores D., Tousignant A., Skipper J. K. and Wibbels T. (1994). Temperature-dependent sex determination in reptiles: proximate mechanisms, ultimate outcomes, and practical applications. Dev. Genet. 15, 297-312. 10.1002/dvg.1020150310 [DOI] [PubMed] [Google Scholar]
- Diczfalusy E. (1969). Steroid metabolism in the human foeto-placental unit. Acta Endocrinol. 61, 649-664. 10.1530/acta.0.0610649 [DOI] [PubMed] [Google Scholar]
- Ewert M. A. and Legler J. M. (1978). Hormonal induction of oviposition in turtles. Herpetologica 34, 314-318. [Google Scholar]
- Fowden A. L., Li J. and Forhead A. J. (1998). Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc. Nutr. Soc. 57, 113-122. 10.1079/PNS19980017 [DOI] [PubMed] [Google Scholar]
- Goodman H. M. (2003). Basic Medical Endocrinology. China: Academic Press. [Google Scholar]
- Goy R. W. and McEwen B. S. (1980). Sexual Differentiation of the Brain. Cambridge, MA: MIT Press. [Google Scholar]
- Greenbaum E. (2002). A standardized series of embryonic stages for the emydid turtle Trachemys scripta. Can. J. Zool. 80, 1350-1370. 10.1139/z02-111 [DOI] [Google Scholar]
- Groothuis T. G. G. and Schwabl H. (2008). Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1647-1661. 10.1098/rstb.2007.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T. G. G., Müller W., Von Engelhardt N., Carere C. and Eising C. (2005). Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329-352. 10.1016/j.neubiorev.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Haccard O., Dupré A., Liere P., Pianos A., Eychenne B., Jessus C. and Ozon R. (2012). Naturally occurring steroids in Xenopus oocyte during meiotic maturation. Unexpected presence and role of steroid sulfates. Mol. Cell. Endocrinol. 362, 110-119. 10.1016/j.mce.2012.05.019 [DOI] [PubMed] [Google Scholar]
- Hsu B.-Y., Dijkstra C. and Groothuis T. G. G. (2016). No escape from mother's will: effects of maternal testosterone on offspring reproductive behaviour far into adulthood. Anim. Behav. 117, 135-144. 10.1016/j.anbehav.2016.05.004 [DOI] [Google Scholar]
- Les H. L., Paitz R. T. and Bowden R. M. (2009). Living at extremes: development at the edges of viable temperature under constant and fluctuating conditions. Physiol. Biochem. Zool. 82, 105-112. 10.1086/590263 [DOI] [PubMed] [Google Scholar]
- Levitz M., Condon G. P., Money W. L. and Dancis J. (1960). The relative transfer of estrogens and their sulfates across the guinea pig placenta: sulfurylation of estrogens by the placenta. J. Biol. Chem. 235, 973-977. [PubMed] [Google Scholar]
- Loriaux D. L., Ruder H. J., Knab D. R. and Lispett M. B. (1972). Estrone sulfate, estrone, estradiol and estriol plasma levels in human pregnancy. J. Clin. Endocrinol. Metab. 35, 887-891. 10.1210/jcem-35-6-887 [DOI] [PubMed] [Google Scholar]
- Miki Y., Nakata T., Suzuki T., Darnel A. D., Moriya T., Kaneko C. and Sasano H. (2002). Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J. Clin. Endocrinol. Metab. 87, 5760-5768. 10.1210/jc.2002-020670 [DOI] [PubMed] [Google Scholar]
- Moisiadis V. G. and Matthews S. G. (2014). Glucocorticoids and fetal programming part 2: mechanisms. Nat. Rev. Endocrinol. 10, 403-411. 10.1038/nrendo.2014.74 [DOI] [PubMed] [Google Scholar]
- Moore M. C. and Johnston G. I. H. (2008). Toward a dynamic model of deposition and utilization of yolk steroids. Integr. Comp. Biol. 48, 411-418. 10.1093/icb/icn079 [DOI] [PubMed] [Google Scholar]
- Mortaud S., Donsez-Darcel E., Roubertoux P. L. and Degrelle H. (1996). Murine steroid sulfatase gene expression in the brain during postnatal development and adulthood. Neurosci. Lett. 215, 145-148. 10.1016/0304-3940(96)12944-X [DOI] [PubMed] [Google Scholar]
- Mueller J. W., Gilligan L. C., Idkowiak J., Arlt W. and Foster P. A. (2015). The regulation of steroid action by sulfation and desulfation. Endocr. Rev. 36, 526-563. 10.1210/er.2015-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz R. T. and Bowden R. M. (2008). A proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. Integr. Comp. Biol. 48, 419-427. 10.1093/icb/icn034 [DOI] [PubMed] [Google Scholar]
- Paitz R. T. and Bowden R. M. (2009). Rapid decline in the concentrations of three yolk steroids during development: Is it embryonic regulation? Gen. Comp. Endocrinol. 161, 246-251. 10.1016/j.ygcen.2009.01.018 [DOI] [PubMed] [Google Scholar]
- Paitz R. T. and Bowden R. M. (2011). Biological activity of oestradiol sulphate in an oviparous amniote: Implications for maternal steroid effects. Proc. R. Soc. Lond. [Biol]. 278, 2005-2010. 10.1098/rspb.2010.2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz R. T. and Bowden R. M. (2013). Sulfonation of maternal steroids is a conserved metabolic pathway in vertebrates. Integr. Comp. Biol. 53, 895-901. 10.1093/icb/ict027 [DOI] [PubMed] [Google Scholar]
- Paitz R. T. and Bowden R. M. (2015). The in ovo conversion of oestrone to oestrone sulfate is rapid and subject to inhibition by Bisphenol A. Biol. Lett. 11, 20140946 10.1098/rsbl.2014.0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz R. T. and Casto J. M. (2012). The decline in yolk progesterone concentrations during incubation is dependent on embryonic development in the European starling. Gen. Comp. Endocrinol. 176, 415-419. 10.1016/j.ygcen.2011.12.014 [DOI] [PubMed] [Google Scholar]
- Paitz R. T., Bowden R. M. and Casto J. M. (2011). Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris). Proc. R. Soc. Lond. [Biol]. 278, 99-106. 10.1098/rspb.2010.0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz R. T., Sawa A. R. and Bowden R. M. (2012). Characterizing the metabolism and movement of yolk estradiol during embryonic development in the red-eared slider (Trachemys scripta). Gen. Comp. Endocrinol. 176, 507-512. 10.1016/j.ygcen.2011.10.009 [DOI] [PubMed] [Google Scholar]
- Reed M. J., Purohit A., Woo L. W. L., Newman S. P. and Potter B. V. L. (2005). Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr. Rev. 26, 171-202. 10.1210/er.2004-0003 [DOI] [PubMed] [Google Scholar]
- Schwabl H. (1993). Yolk is a source of maternal testosterone for developing birds. Proc. Natl. Acad. Sci. USA 90, 11446-11450. 10.1073/pnas.90.24.11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strott C. A. (2002). Sulfonation and molecular action. Endocr. Rev. 23, 703-732. 10.1210/er.2001-0040 [DOI] [PubMed] [Google Scholar]
- Vassallo B. G., Paitz R. T., Fasanello V. J. and Haussmann M. F. (2014). Glucocorticoid metabolism in the in ovo environment modulates exposure to maternal corticosterone in Japanese quail embryos (Coturnix japonica). Biol. Lett. 10, 20140502 10.1098/rsbl.2014.0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt N., Carere C., Dijkstra C. and G. G. and Groothuis T. (2006). Sex-specific effects of yolk testosterone on survival, begging and growth of zebra finches. Proc. R. Soc. Lond. [Biol]. 273, 65-70. 10.1098/rspb.2005.3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt N., Henriksen R. and Groothuis T. G. G. (2009). Steroids in chicken egg yolk: Metabolism and uptake during early embryonic development. Gen. Comp. Endocrinol. 163, 175-183. 10.1016/j.ygcen.2009.04.004 [DOI] [PubMed] [Google Scholar]
- Williams T. D. (2012). Physiological Adaptations for Breeding in Birds. Princeton, NJ: Princeton University Press. [Google Scholar]
- Williams T. D. and Groothuis T. G. G. (2015). Egg quality, embryonic development, and post-hatching phenotype: an integrated perspective. In Nests, Eggs, and Incubation: New Ideas about Avian Reproduction (ed. Deeming D. C. and Reynolds S. J.), pp. 113-126. Oxford: Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.