Fig. 1.
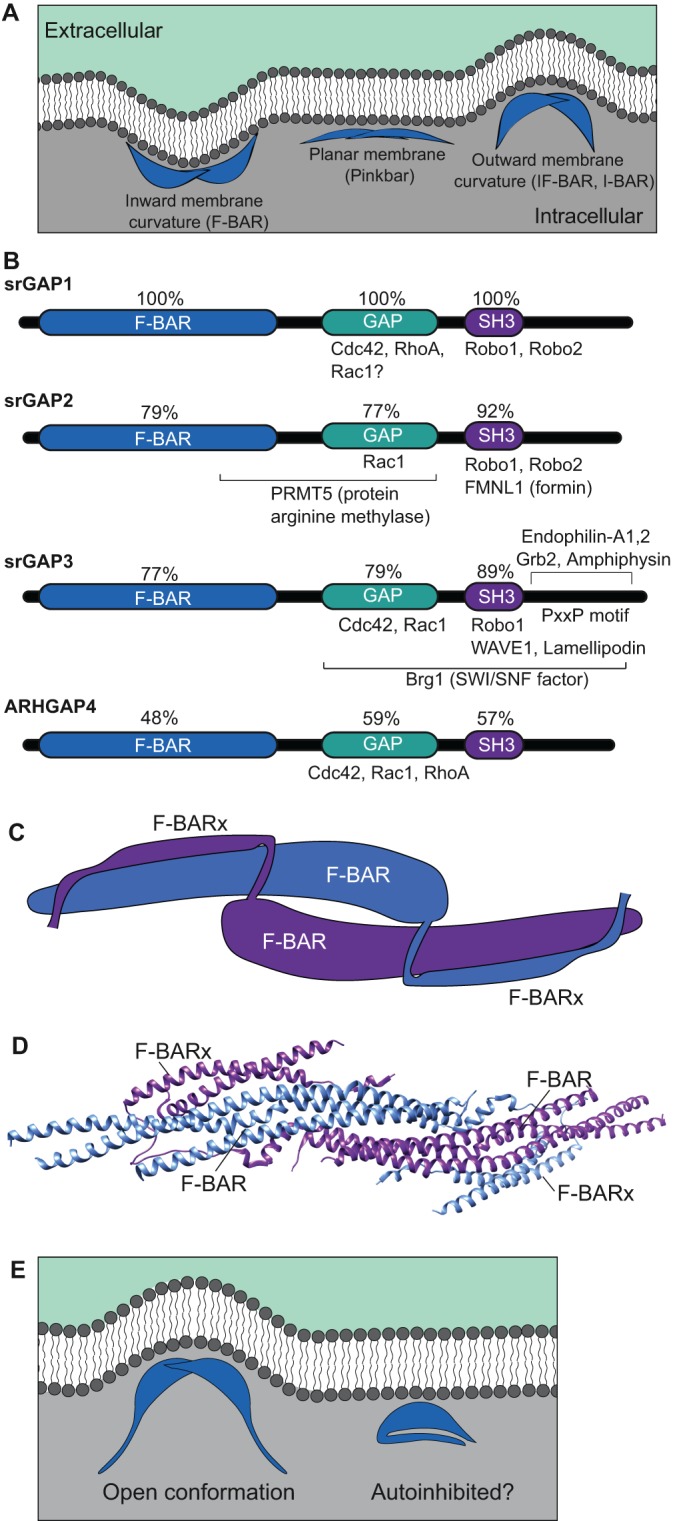
srGAPs are BAR domain proteins with characteristic functional domains. (A) BAR domains (blue) allow proteins to dimerize and interact with specific phospholipids (dark gray) at the membrane. FCH-Bin, Amphiphysin, Rvs (F-BAR) domains interface with membranes through their concave surfaces. Inverse BAR (I-BAR) and inverse F-BAR (IF-BAR) domains associate with membranes through their convex surfaces, generally interfacing with evaginations. Pinkbar associates with non-curved planar membranes through its convex surface. (B) Domain structure of srGAPs. Vertebrate srGAPs have three highly conserved domains: the lipid-binding F-BAR domain, a GAP domain that serves to inactivate Rho GTPase family members, and the SH3 domain, which is important for multiple protein-protein interactions. Regions mediating known binding interactions are indicated for each family member; see main text for references. (C) Diagrammatic representation of two srGAP2 monomers within an inferred homodimer, based on a rendering of PDB 5I6J, as originally reported in Sporny et al. (2017). The F-BAR and extended F-BAR domains (F-BARx) interact to stabilize the homodimer. (D) Structure of the presumed homodimer based on crystallographic data (PDB 5I6J; rendered using UCSF Chimera). (E) Possible model for autoinhibition of the F-BAR domain. When the F-BAR domains of two srGAP proteins homodimerize, membrane evaginations are generated (left).
