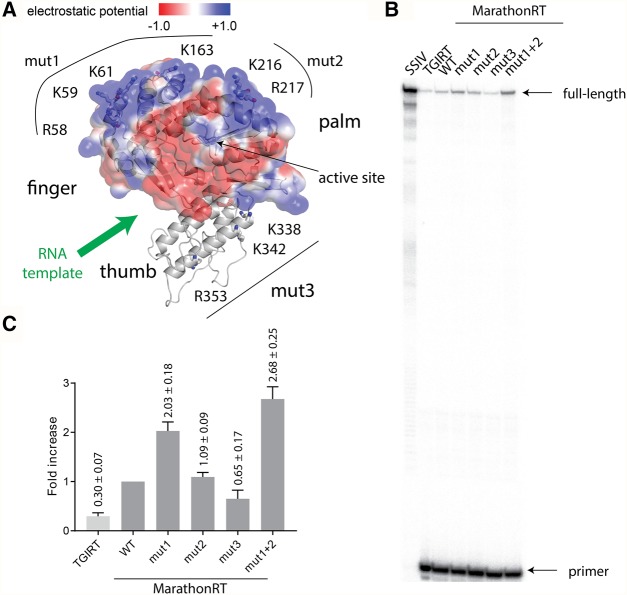
FIGURE 6.
Positively charged RNA binding surface affects RT efficiency on lncRNA RepA D1. (A) Three-dimensional model (generated as described for Fig. 5A) showing the positively charged RNA binding surface (blue) on the RT domain of MarathonRT. The electrostatic surface potential of the RT domain was calculated using APBS (Baker et al. 2001) and PDB2PQR (Dolinsky et al. 2007) and is represented as a transparent surface. Residues that are mutated in mut1, mut2, and mut3 constructs were shown as sticks. (B) Gel showing the RT products produced by SSIV, TGIRT, and different constructs of MarathonRT using RepA D1 (Liu et al. 2017) as template under multi-turnover conditions. (C) Fold increase in the primer incorporation efficiency for various enzymes relative to WT MarathonRT. Primer incorporation efficiency is the ratio of all extension products relative to the total amount of primer in the reaction (equal to all extension products plus unincorporated primers).