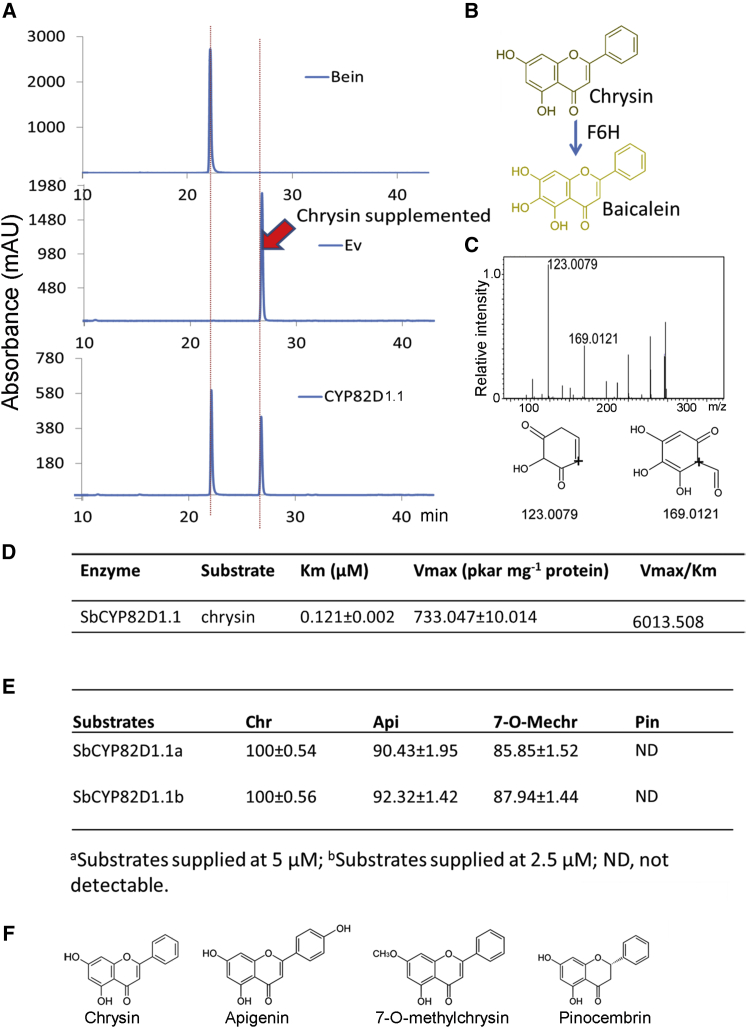
Figure 2.
Characterization of SbCYP82D1.1 Enzyme Activity.
(A) Assays of activity in yeast in vivo. HPLC analysis of yeast samples incubated with chrysin: top, baicalein standard; middle, yeast with empty vector (Ev); bottom, yeast expressing SbCYP82D1.1, where a new peak with the same retention time as baicalein was found. Bein, baicalein.
(B) The proposed reaction catalyzed by SbCYP82D1.1.
(C) MS2 and fragmentation patterns of the new compound produced by SbCYP82D1.1 when expressed in yeast, which was identical to baicalein.
(D) Kinetic analyses of CYP82D1.1 determined in vitro following expression in yeast; each dataset represents the mean ± SE from triplicate measurements.
(E) Relative turnover rate of CYP82D1.1 with apigenin, 7-O-methylchrisin, or pinocembrin used as substrates. Chr, chrysin; Api, apigenin; 7-O-Mechr, 7-O-methylchrysin; Pin, pinocembrin. ND, not detected; each dataset represents the mean ± SE from triplicate measurements, for 5 μM chr, 100% = 707.138 pkat mg−1 protein; for 2.5 μM chr, 100% = 702.098 pkat mg−1 protein.
(F) Structures of the substrates used in the CYP82D1.1 in vitro assays.