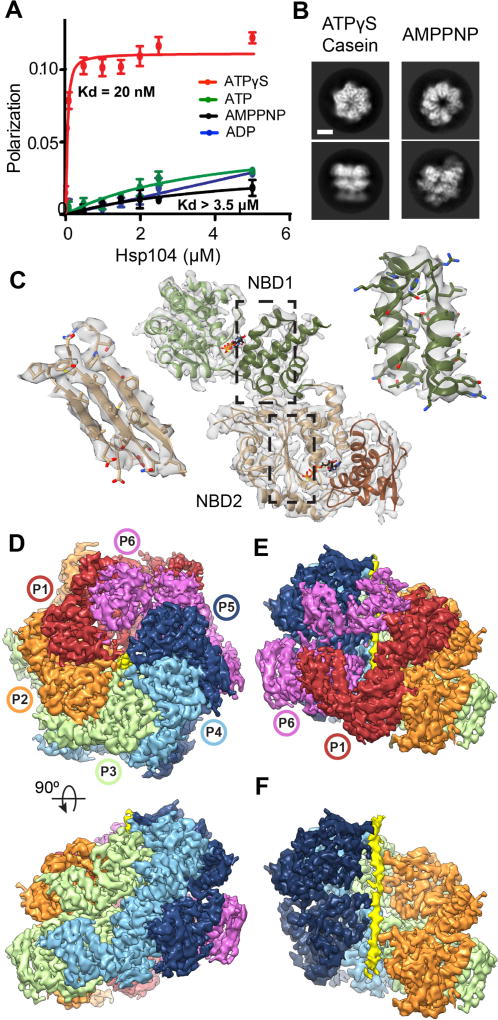
Fig. 1. Substrate-bound Hsp104:casein closed complex.
(A) FITC-casein binding analysis, measured by fluorescence polarization in the presence of: ATPγS (red), ATP (green), AMPPNP (black), and ADP (blue) (values = mean ±SD, n=3). (B) Representative top and side-view 2D class averages comparing Hsp104-ATPγS:casein closed state and Hsp104-AMPPNP open state(9) (scale bar equals 50 Å). (C) Atomic model and segmented map of the AAA+ small (NBD1, green) and large subdomains (NBD2, brown). (D) Final reconstruction of Hsp104:casein segmented by protomers (P1–P6) and substrate (yellow). (E) Side view of the mobile protomer face (P1 and P6). (F) Channel view showing substrate polypeptide density (yellow).