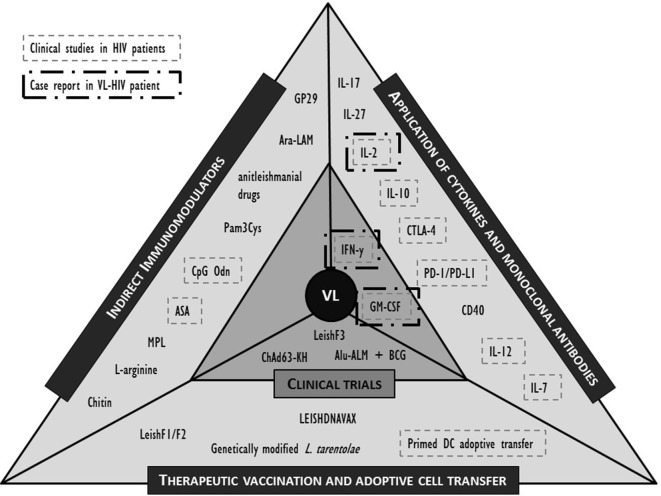
Figure 2.
Overview of described clinical and preclinical immunomodulatory interventions in human visceral leishmaniasis (VL) and their application in (VL)-human immunodeficiency virus (HIV) (co)infection. IL, interleukin; IFN, interferon; PD-(L)1, programmed cell death-(ligand)1; GM-CSF, granulocyte–macrophage colony-stimulating factor; CTLA, cytotoxic T lymphocyte-associated molecule; CD, cluster of differentiation; BCG, Bacillus Calmette–Guérin; Alu-ALM, aluminum hydroxide precipitated autoclaved L. major; DC, dendritic cell; GP, Glycoprotein; Ara-LAM, arabinosylated lipoarabinomannan; Pam3Cys, synthetic bacterial lipopeptide; CpG Odn, CpG oligodeoxynucleotides; ASA, acetyl salicylic acid; MPL, monophosphoryl lipid.