Fig. 6.
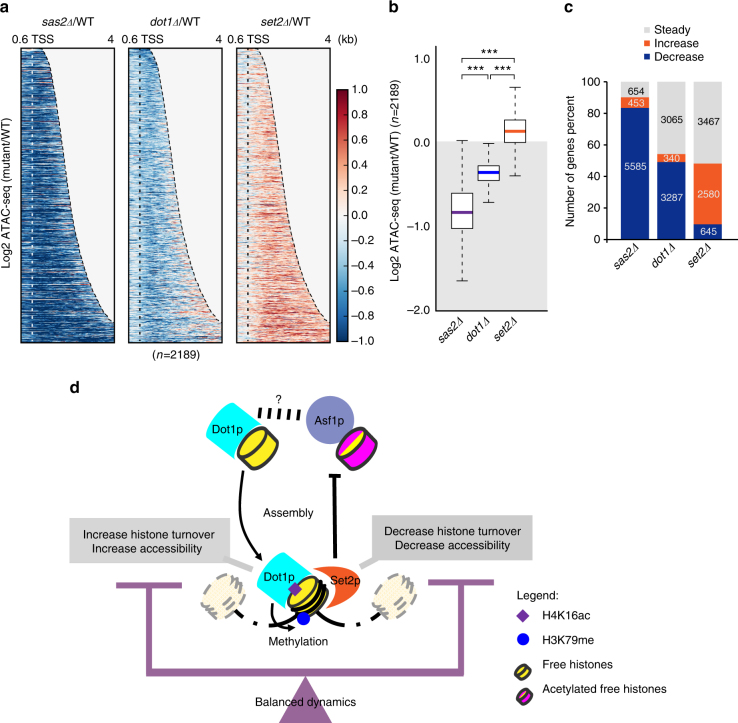
Dot1p facilitates nucleosome accessibility in the transcribed region of genes. a Heatmap for the log2 fold change of the ATAC-seq signal in sas2Δ (left), dot1Δ (center), and set2Δ (right) cells versus wild type. Increased accessibility is indicated in red, and decreased accessibility is indicated in blue. Genes were ordered by their accessibility difference in dot1Δ versus wild-type cells (n = 2189). Data were obtained from biological duplicates. The y-axis indicates each genes and the x-axis indicates distance from TSS. The genes in heatmap was arranged in order of gene length. The color bar represents value of log2 mutant compared to wild-type (color red: >0, color blue: <0). The dotted lines indicate the TSS. All values were normalized to the read count, and the value of the region exceeding the TES was treated as 0. b A boxplot of ATAC signal log2 fold changes in mutants over wild type. The value of ATAC signal implies chromatin accessibility. The values of the y-axis indicate log2 fold change level of ATAC-seq value in each sas2Δ (purple), dot1Δ (blue), and set2Δ (orange) mutant over wild-type cells. ***P-value <0.001, calculated by Wilcoxon and Mann–Whitney tests, and the shadowed box represents negative values. c Stacked percent graph for the difference in accessibility between mutant and wild-type cells. Log2 fold changes between mutants (sas2Δ, dot1Δ, and set2Δ) and wild-type on transcribed regions were analyzed using Bwtool and the following classification criteria: increased (orange), log2 fold change >0.2; decrease (blue), log2 fold change <−0.2; steady (gray), −0.2 <log2 fold change <0.2. d The proposed model. Dot1p binds the nucleosome with the support of H4K16ac (purple diamond), and increases histone turnover and accessibility via its inherent histone chaperone activity (nucleosome binding). Conversely, Set2 acts to decrease histone turnover and accessibility, thereby balancing nucleosome dynamics. Dot1p methylates H3K79 by nucleosome binding (blue circle), but this activity is independent of its involvement in balancing nucleosome dynamics