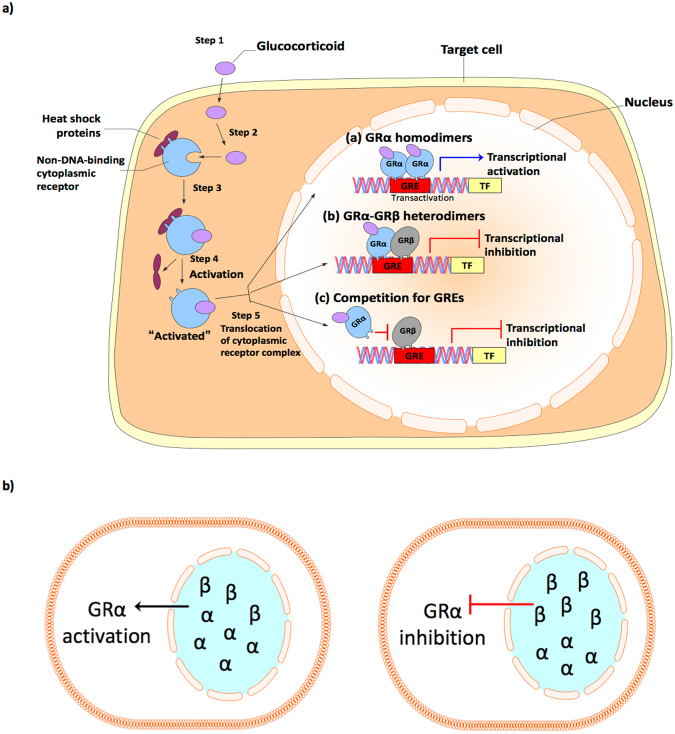
Figure 6.
Mechanism of action of GRβ. (a) The classical pharmacological and physiological actions of GCs are mediated by the GR isoform GRα. In the absence of ligand (i.e GCs), GRα predominantly resides in the cytoplasm of cells as part of a large multiprotein complex that includes chaperone proteins (hsp90, hsp70, and p23) and immunophilins (FKBP51 and FKBP52), maintaining the high-affinity ligand binding GR confirmation. Upon binding ligand, GRα undergoes a conformational change, resulting in the dissociation of the multiprotein complex (Steps 1, 2, and 3). Structural reorganization of the GRα protein exposes nuclear localization signals, and the ligand-bound GRα is rapidly translocated by microtubule motor proteins along the microtubules into the nucleus through nuclear pores (Steps 4 and 5). Once inside the nucleus, GRα forms homodimers that bind directly to GREs and stimulate target gene expression (a). The GRβ isoform acts as a natural dominant negative inhibitor of GRα-induced transactivation of glucocorticoid-responsive genes. GRβ antagonizes GRα activities by forming heterodimers with GRα inhibiting its transcriptional activities (b), and by competition for GC response elements (c). (b) GRβ overexpression in the cell leads to GRα inhibition and make cells more resistance to GCs.