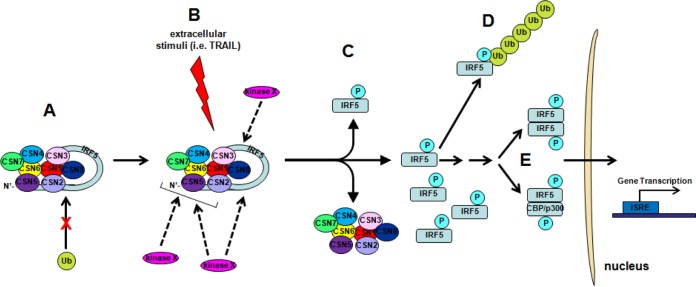
FIG 9.
Model of IRF5 stabilization and activation. (A) In unstimulated cells, cytoplasmic IRF5 monomers constitutively interact with CSN subunits at both the carboxyl- and amino-terminal regions, thus masking the DNA binding domain of IRF5 by the AID. This leads to the effective blocking of IRF5 transcriptional activity in unstimulated cells and protects IRF5 from degradation by the ubiquitin-proteasome pathway. (B) Upon stimulation with an activation trigger such as TRAIL, an as yet to be identified kinase(s) (kinase x) uses the CSN complex as a scaffold and phosphorylates IRF5 and/or CSN subunits that in turn phosphorylate IRF5. (C) Phosphorylation of IRF5 results in structural changes that lead to dissociation from the CSN complex. (D) Loss of the CSN/IRF5 interaction results in K48-linked ubiquitination of IRF5 and degradation by the ubiquitin-proteasome pathway. (E) Some “activated” IRF5 forms homodimers or heterodimers with other proteins, such as CBP/p300, and translocates to the nucleus, where it binds to promoters of target genes and regulates their transcription.