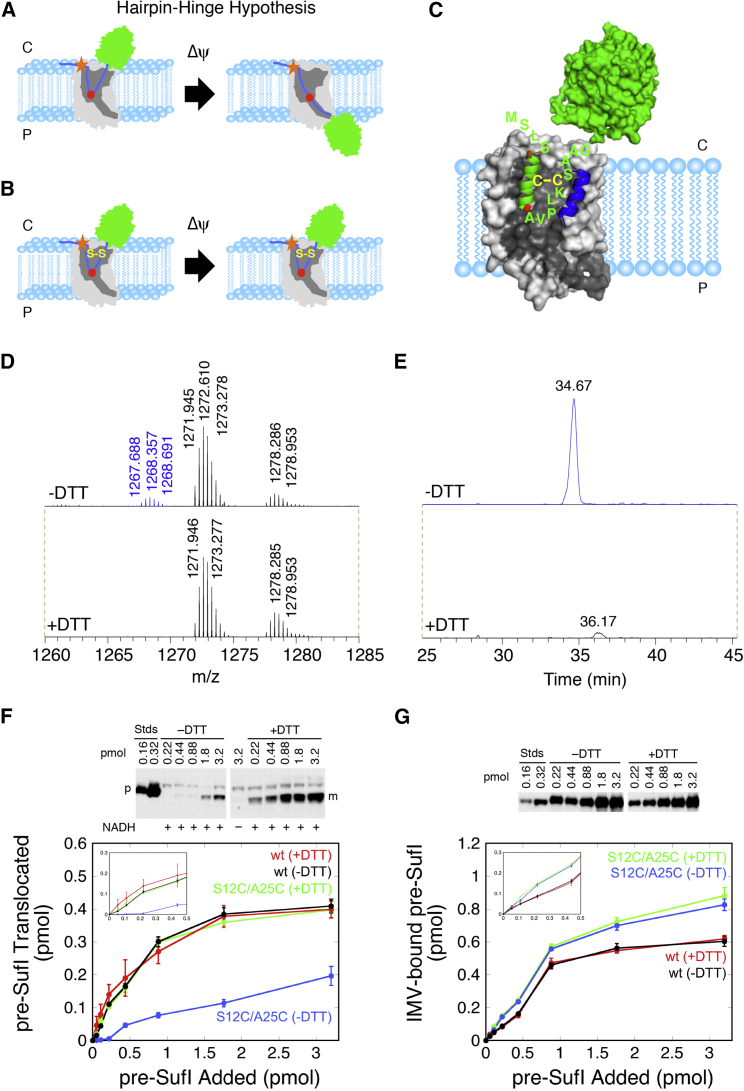
Figure 8.
Testing the hairpin-hinge hypothesis with an internally cross-linked signal peptide. (A) Shown here is the hairpin-hinge hypothesis. During translocation of the mature domain (green) from the cytoplasm (C) to the periplasm (P), the hairpin formed by the signal peptide (blue) is hypothesized to open via a hinge (high flexibility) region (red) at the tip of the hairpin. The position of the RR-motif is indicated (orange star). (B) Shown here is how a disulfide can block unhinging of the signal peptide hairpin. According to the hairpin-hinge hypothesis, an internally disulfide-cross-linked signal peptide hairpin cannot unhinge under energized conditions, thus blocking translocation. Binding is predicted to be unaffected (compare with (A)). (C) Shown here is the predicted interaction of the oxidized double-cysteine mutant pre-SufI(S12C/A25C) with the TatBC complex (compare with Fig. 7B). (D) Shown here is liquid chromatography-tandem mass spectrometry analysis of trypsin-digested as-isolated pre-SufI(S12C/A25C). The peak centered at m/z = 1268.357 is consistent with a +3H ion consisting of two peptides (amino acids 12–24 and 25–43) linked by a disulfide bond between S12C and A25C. Trypsin digests peptides after lysine and arginine residues—in particular, digesting pre-SufI(S12C/A25C) after R11, K24, and R43. Under +DTT conditions, the m/z = 1268.357 peak was substantially diminished, consistent with reduction of the disulfide. Other peaks were unchanged and used for calibration. CID analysis confirmed the identity of the two indicated peptides (Fig. S9A). (E) Shown here is extracted ion chromatography of the disulfide-linked peptide identified in (D). The ion intensity was reduced by ∼97% under +DTT conditions. (F) Shown here is the transport yield of pre-SufI(S12C/A25C). After enriching the disulfide-linked form of pre-SufI(S12C/A25C) to ∼60% (see Materials and Methods; Fig. S9B), transport assays with Tat++ IMVs were performed over a range of initial precursor concentrations. In the presence of DTT, pre-SufI(S12C/A25C) transported similarly to the pre-SufI(497C) (wt) protein. However, in the absence of DTT, pre-SufI(S12C/A25C) transport was substantially diminished. This effect was largest at lower precursor concentrations, suggesting that at higher concentrations more of the un-cross-linked protein is transported (p, precursor; m, mature). (G) Shown here is membrane-bound pre-SufI(S12C/A25C). The high amount of pre-SufI(S12C/A25C) bound to Tat++ IMVs in the presence and absence of DTT indicates that the lower transport yield observed under –DTT conditions in (F) cannot be explained by weaker membrane binding interactions. The disulfide cross-linked pre-SufI protein binds to Tat translocons with similar affinity as the wt protein (Fig. S10).