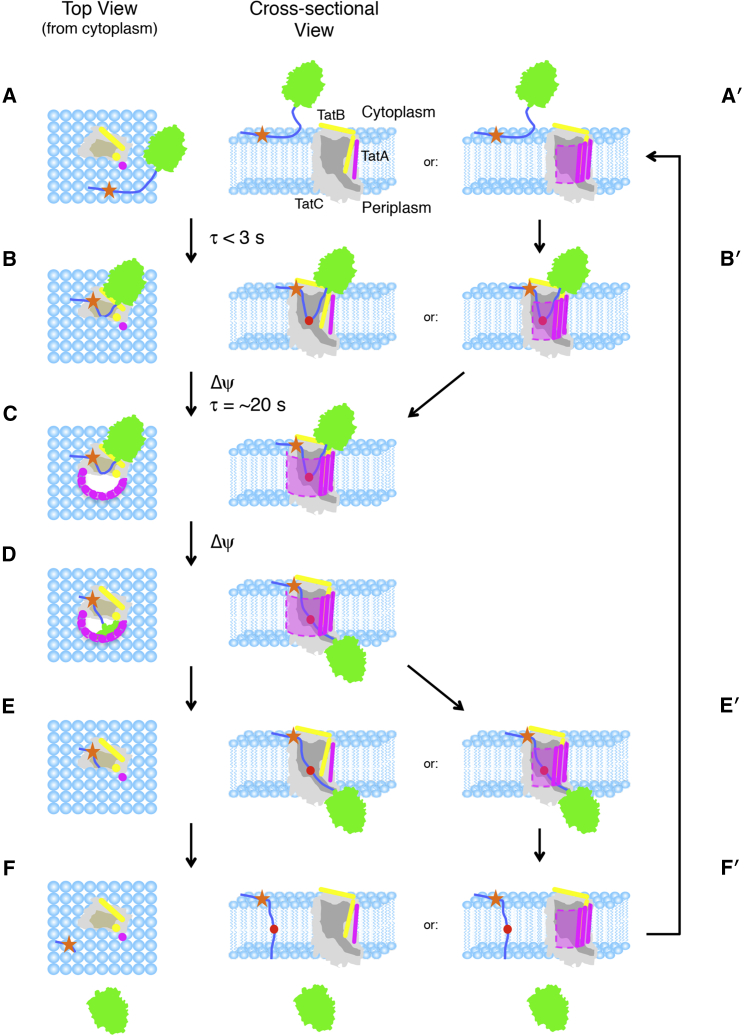
Figure 9.
Hairpin-hinge model of Tat translocation. (A) The precursor protein (green) binds to the membrane lipids via its signal peptide (dark blue) (34, 36). (B) Binding to the membrane surface, diffusion to the receptor complex, and membrane insertion of the signal peptide hairpin occurs rapidly (<3 s) and is pmf-independent (29). The RR-motif (orange star) interacts with E15 and E103 of TatC (gray) (12) and the C-terminal end of the signal peptide after the hinge (red) interacts with TatB (yellow). The amphipathic helix of TatB likely contacts the mature domain (19). (C) In the presence of a transmembrane electric field gradient (Δψ), TatA (purple) is recruited to the precursor/receptor complex, resulting in formation of a translocation conduit (pink and dashed outline) with a time constant of ∼20 s (15, 29). (D) Unhinging of the signal peptide hairpin allows the mature domain to translocate across the membrane. (E) The translocation conduit disintegrates after transport. When the pmf is collapsed, TatA dissociates with a time constant of ∼10 s (15). (F) The signal peptide is cleaved from the mature domain by signal peptidase and diffuses into the membrane bilayer, where it is subsequently degraded (81). (E′) In a variation of this model, TatA does not completely dissociate from the receptor complex after mature domain translocation. In this scenario, the translocation conduit can be gated (sealed closed) by movement of lipids into the translocation passageway (not shown). (F′) The signal peptide is released through the lateral lipid-filled gate into the membrane. (A′ and B′) In the presence of high precursor concentrations, recruitment of a new cargo molecule occurs before complete pore disassembly. For clarity, the cytoplasmic domains of TatA and TatB are not shown. These domains may play a role in gating the translocation channel and/or the formation of the pore itself.