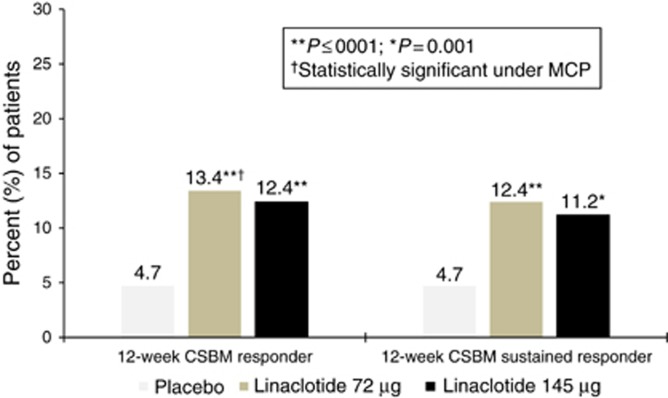
Figure 2.
Percent of patients who were 12-week CSBM overall responders (primary endpoint) and sustained responders (additional endpoint). 12-week CSBM overall responder (primary endpoint): ≥3 CSBMs and an increase of ≥1 CSBM per week (weekly CSBM responder) for ≥9 of the 12 weeks of treatment period (P<0.0001 vs. placebo for both 72 μg and 145 μg doses, however only the 72 μg dose was evaluated under the multiple comparisons procedure (MCP)). Placebo, N=401; Linaclotide 72 μg, N=411; Linaclotide 145 μg, N=411. 12-week CSBM sustained responder (additional endpoint): 12-week CSBM overall responder who meets weekly CSBM responder criteria for ≥3 of the final 4 weeks of the treatment period (P=0.0001 and P=0.0010 vs. placebo for the 72 μg and 145 μg doses, respectively; this analysis was not controlled for multiplicity). Placebo, N=401; Linaclotide 72 μg, N=411; Linaclotide 145 μg, N=411. For both presented analyses, P-values were obtained from the CMH tests controlling for baseline SBM stratum and geographic region.