Abstract
Infection of mice with Sindbis virus (SINV) produces encephalomyelitis and provides a model for examination of the central nervous system (CNS) immune response to alphavirus infection. Clearance of infectious virus is accomplished through a cooperative effort between SINV-specific antibody and IFN-γ, but the regulatory interactions are poorly understood. To determine the effects of IFN-γ on clinical disease and the antiviral immune response, C57BL/6 mice lacking IFN-γ (Ifng−/−) or IFN-γ receptor (Ifngr1−/−) were studied in comparison to WT mice. Maximum production of Ifng mRNA and IFN-γ protein in the CNS of WT and Ifngr1−/− mice occurred 5–7 days after infection, with higher levels of IFN-γ in Ifngr1−/− mice. Onset of clinical disease was earlier in mice with impaired IFN-γ signalling, although Ifngr1−/− mice recovered more rapidly. Ifng−/− and Ifngr1−/− mice maintained body weight better than WT mice, associated with better food intake and lower brain levels of inflammatory cytokines. Clearance of infectious virus from the spinal cords was slower, and CNS, but not serum, levels of SINV-specific IgM, IgG2a and IgG2b were lower in Ifngr1−/− and Ifng−/− mice compared to WT mice. Decreased CNS antiviral antibody was associated with lower expression of mRNAs for B-cell attracting chemokines CXCL9, CXCL10 and CXCL13 and fewer B cells in the CNS. Therefore, IFN-γ signalling increases levels of CNS pro-inflammatory cytokines, leading to clinical disease, but synergistically clears virus with SINV-specific antibody at least in part by increasing chemokine production important for infiltration of antibody-secreting B cells into the CNS.
Keywords: Sindbis virus, viral encephalomyelitis, CNS antibody response, virus clearance, B cells, TNF
Introduction
Mosquito-borne viruses that produce encephalomyelitis are an increasing worldwide concern as viruses and their arthropod vectors expand into new territories (Griffin, 2010a; Gubler, 2002; Lambrechts et al., 2010; van den Hurk et al., 2009; Weaver & Reisen, 2010). The New World alphaviruses, which include eastern, western and Venezuelan equine encephalitis viruses, infect neurons and produce encephalomyelitis with varying fatality rates in dead-end hosts such as humans and horses (Griffin, 2013; Steele et al., 2007). However, humans who survive the initial disease, particularly those infected as infants or children, have a high probability of being left with life-long physical and mental disabilities (Bruyn & Lennette, 1953; Earnest et al., 1971; Finley et al., 1955; Palmer & Finley, 1956; Villari et al., 1995). Currently, there are no treatments beyond supportive care (Griffin, 2010b).
Sindbis virus (SINV) is the prototypic alphavirus, and infection of susceptible mice provides a valuable model for studying the pathogenesis of and host immune response to alphavirus infection of the central nervous system (CNS). When C57BL/6 mice are intracranially infected with the nonfatal TE strain of SINV, infectious virus is cleared from the CNS within 7–10 days, although clearance of viral RNA occurs much more slowly and is not complete (Levine & Griffin, 1992; Metcalf & Griffin, 2011; Tyor et al., 1992). Determining how the immune response clears infectious virus and prevents reactivation is critical to understanding the pathogenesis of viral encephalomyelitis and identifying potential interventions.
As neurons are a valuable yet finite population of cells with little regenerative capacity, clearance of virus requires noncytolytic mechanisms to avoid neurological damage. While initial control of virus replication is dependent on type I IFN (Burdeinick-Kerr et al., 2007; Byrnes et al., 2000; Frolov et al., 2012), the adaptive immune response is responsible for later virus clearance. Previous work using severe combined immunodeficiency mice, which are incapable of clearing SINV, has shown that treatment with hyperimmune serum results in successful clearance of infectious virus from all regions of the CNS, indicating a central role for antibody in clearance (Burdeinick-Kerr et al., 2007; Levine et al., 1991). However, virus clearance from the brain stem and spinal cord can also occur in the absence of antibody and is dependent on the cytokine IFN-γ produced by both CD8+ and CD4+ T cells (Binder & Griffin, 2001). Mice that lack both antibody and IFN-γ clear infectious virus less well than mice deficient in either antibody or IFN-γ (Burdeinick-Kerr et al., 2007), indicating synergistic cooperation between the two in facilitating virus clearance. However, the mechanisms through which this occurs have not been elucidated.
IFN-γ exerts its antiviral effects primarily by interacting with the IFN-γ receptor and inducing production of IFN-stimulated genes (ISGs). The IFN-γ receptor, present on neurons, consists of a heterotetramer of the ligand-binding IFN-γR1 and signalling IFN-γR2 subunits (Rottenberg & Kristensson, 2002; Tau & Rothman, 1999). IFN-γ signalling activates Jak kinases to phosphorylate STAT proteins, particularly STAT1, which dimerize, translocate to the nucleus and bind to gamma-associated site elements in the promoters of ISGs (Burdeinick-Kerr et al., 2009). IFN-γ signalling activates over 200 genes with varying functions, resulting in multifaceted modulation of the immune response (Farrar & Schreiber, 1993; Tau & Rothman, 1999), and plays a vital role in the clearance of other neurotropic infections, including mouse hepatitis virus (MHV), Borna disease virus, herpes simplex virus (HSV), West Nile virus and Listeria monocytogenes (Geiger et al., 1995; Hallensleben & Staeheli, 1999; Jin et al., 2004; Klein & Diamond, 2008; Pearce et al., 1994). These immunomodulatory functions include effects on immune cell trafficking, T helper cell differentiation, antigen presentation, cytokine and chemokine production, IgG class switching and macrophage activation (Farrar & Schreiber, 1993; Rottenberg & Kristensson, 2002; Samuel, 2001), but the processes by which IFN-γ specifically regulates the disease manifestations and the immune response during alphavirus encephalomyelitis have not yet been characterized. In these studies, we used mice deficient in either IFN-γ (Ifng−/−) or IFN-γR1 (Ifngr1−/−) and show that lack of IFN-γ signalling leads to not only less severe clinical disease, but also less efficient entry of antibody-secreting B cells into the CNS and slower virus clearance from the spinal cord.
Results
IFN-γ production in the CNS during infection
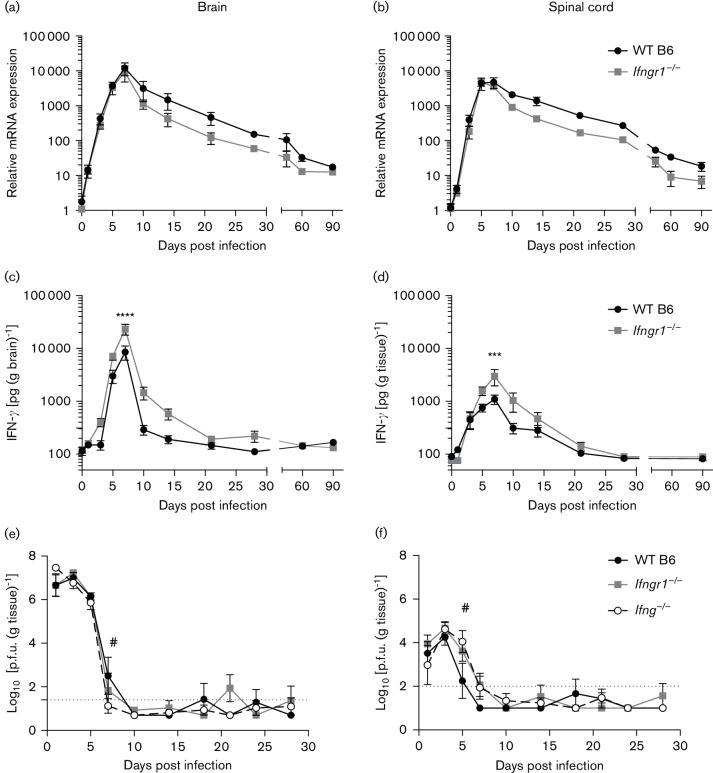
To examine the role IFN-γ plays in the immunopathogenesis and clearance of alphavirus infection of the CNS, production of IFN-γ in the brain and spinal cord was first examined. Relative to 0 days post-infection (DPI) controls, mRNA levels in WT B6 mouse brains and spinal cords increased sharply during SINV infection, peaking at 5–7 DPI (Fig. 1a, b). Expression then slowly decreased, but had not returned to baseline levels at 90 DPI. Ifng expression in Ifngr1−/− mice was similar to WT B6 mice. IFN-γ protein production, as measured by enzyme immunoassay (EIA), also sharply increased in the brain and spinal cord following SINV infection, peaking at 7 DPI in WT B6 mice before decreasing to below detectable limits by 10 DPI (Fig. 1c, d). In contrast to mRNA expression, amounts of IFN-γ protein in the CNS differed between WT B6 and Ifngr1−/− mice (Fig. 1c), with the brains of Ifngr1−/− mice having higher peak levels at 7 DPI. Though the difference in IFN-γ production over time in the spinal cord between WT B6 and Ifngr1−/− mice was not significant (Fig. 1d), peak production at 7 DPI was higher in Ifngr1−/− mice. The decrease in IFN-γ production was slower in Ifngr1−/− mice, with protein still detectable at 14 DPI in both the brain and spinal cord. These data suggest that while mRNA expression of IFN-γ does not differ in the CNS between WT B6 and Ifngr1−/− during SINV infection, impaired IFN-γ signalling results in either increased protein production or decreased protein consumption.
Fig. 1.
Ifng mRNA expression and IFN-γ protein production in WT B6 mice and mice with impaired IFN-γ signalling. (a, b) Ifng mRNA expression determined by quantitative real-time PCR (qRT-PCR) in SINV-infected WT B6 and Ifngr1−/− mouse brains (a) and spinal cords (b) and normalized to 0 DPI control mice of each strain. Data are represented as mean±sem for three to seven mice per strain per time point. (c, d) IFN-γ protein production was measured by EIA in SINV-infected WT and Ifngr1−/−mouse brains (c) and spinal cords (d). Data are presented as mean picograms IFN-γ per gram tissue±sem for 3–11 mice per strain per time point except for 90 DPI Ifngr1−/− spinal cords, where n=2; ***P<0.001, ****P<0.0001, Bonferroni’s multiple comparisons test. (e, f) Infectious virus titres were measured in WT B6, Ifngr1−/− and Ifng−/− mouse brains (e) and spinal cords (f) by plaque assay. Data are presented as the mean±sem for three to eight mice per strain per time point, except for 24 DPI WT B6, where n=2; dotted line represents the limit of detection for the assay; #P<0.05, WT B6 vs Ifng−/−, Tukey’s multiple comparisons test.
Clearance of infectious virus from the brain and spinal cord
Levels of infectious virus in the brains (Fig. 1e) and spinal cords (Fig. 1f) of WT B6, Ifngr1−/− and Ifng−/− mice measured by plaque assay peaked with similar titres at 1–3 DPI. Clearance from brain was similar, but complete clearance from the spinal cord was delayed in the absence of IFN-γ signalling. While infectious virus titres were below detectable levels in WT B6 spinal cords by 7 DPI, infectious virus was still detected in Ifngr1−/− and Ifng−/− mice. In both tissues of all three strains, infectious virus was periodically detected in individual mice through 28 DPI, but only consistently detectable in the brains of Ifngr1−/−mice at 21 DPI. This is likely due to virus reactivation, but could reflect persistence of infectious virus in individual mice. These data show that IFN-γ signalling is important in clearance of infectious virus, particularly from the spinal cord, and that for 2–3 weeks after initial clearance, detectable replicating virus is common.
Clinical manifestations of alphavirus encephalomyelitis
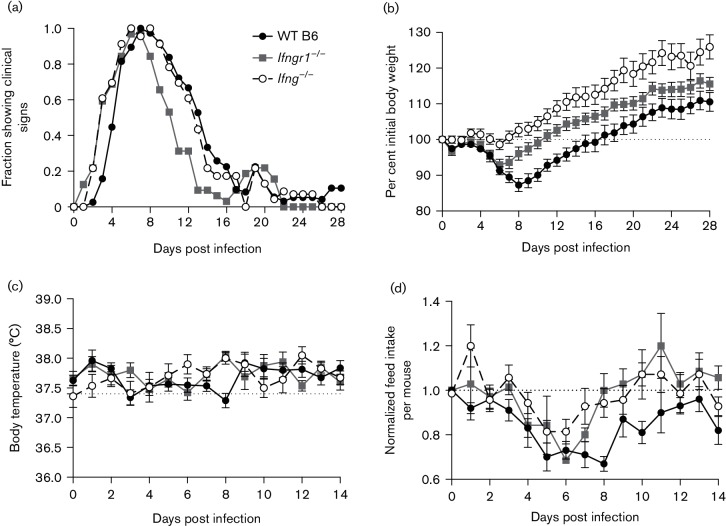
To assess the clinical disease produced by nonfatal SINV infection in Ifngr1−/− and Ifng−/− mice compared to WT B6 mice, animals were weighed and evaluated for signs of encephalomyelitis (a combination of abnormal posture and gait) daily. Ifngr1−/− and Ifng−/− mice developed clinical signs earlier than WT B6 mice (Fig. 2a; median of 3 vs 5 DPI). Almost all mice showed clinical signs by 6 DPI, but Ifngr1−/− mice recovered earlier than Ifng−/− or WT B6 mice (median 11 DPI vs 13 DPI vs 14 DPI for Ifngr1−/−, Ifng−/− and WT B6 mice, respectively). In all three strains, approximately 20 % redeveloped clinical signs from 18 to 21 DPI.
Fig. 2.
Clinical disease in SINV-infected WT B6, Ifngr1−/− and Ifng−/− mice. (a) SINV-infected WT B6, Ifngr1−/− and Ifng−/− mice were evaluated daily for the presence of clinical disease, with data presented as the proportion of mice showing clinical signs each day for 23–38 mice per strain combined from three to five independent experiments. (b) Body weight was measured daily and normalized to the body weight at 0 DPI, with data represented as the mean±sem for 23–38 mice per strain combined from three to five independent experiments; dotted line indicates initial body weight. (c) Body temperature was measured rectally daily, with data represented as the mean±sem for eight mice per strain; dotted line indicates reported normal rectal temperature for mice. (d) Daily feed consumption was measured for two or three mice housed per cage, with feed intake of SINV-infected mice normalized first to that of mock-infected mice for each strain for each day and then to the feed intake at 0 DPI for that cage; data are presented as the mean amount of consumed feed±sem for 7–10 cages combined from two independent experiments; dotted line indicates baseline feed intake.
Significant strain-dependent differences in body weight change were seen during infection (Fig. 2b; P<0.01, two-way ANOVA). WT B6 mice lost approximately 13 % of their initial body weight, reaching a nadir at 8 DPI, before recovering and surpassing their 0 DPI body weight by 17 DPI. In contrast, Ifngr1−/− and Ifng−/− mice lost only an average of 7 and 1 % of their initial body weight, respectively, with a nadir at 6 DPI before recovering and surpassing their 0 DPI body weight by 11 and 7 DPI, respectively. While the rate of weight gain following recovery was approximately the same among strains, by 28 DPI, Ifng−/− mice were significantly heavier than the WT B6 and Ifngr1−/− mice (P<0.0001 and P<0.01 vs WT B6 and Ifngr1−/−, respectively, Tukey’s multiple comparisons test). No difference in body weights was found in mock-infected mice. Prior to infection, WT B6 mice weighed slightly more than Ifngr1−/− mice, but not Ifng−/− mice (mean body weight 17.9, 16.9 and 17.6 g for WT B6, Ifngr1−/− and Ifng−/−, respectively).
To determine the cause of the weight loss in SINV-infected mice, two parameters were measured: body temperature and feed intake. Body temperature did not increase or decrease with SINV infection, fluctuating only about 0.5 °C throughout the first 14 DPI (Fig. 2c). When normalized to mock-infected strain controls at each day and then to the amount of food consumed at 0 DPI, all three strains decreased their feed intake during the first week following infection (Fig. 2d). However, while Ifngr1−/− and Ifng−/− mice increased the amount of feed they consumed starting at 7 DPI, WT B6 mice continued to show a decrease in daily consumption through 8 DPI. These patterns coincided with the times during which mice of each strain lost or regained body weight. Based on these data, the differences in body weight change among strains can likely be attributed to differences in appetite suppression following infection.
Cytokine expression and production in the brain
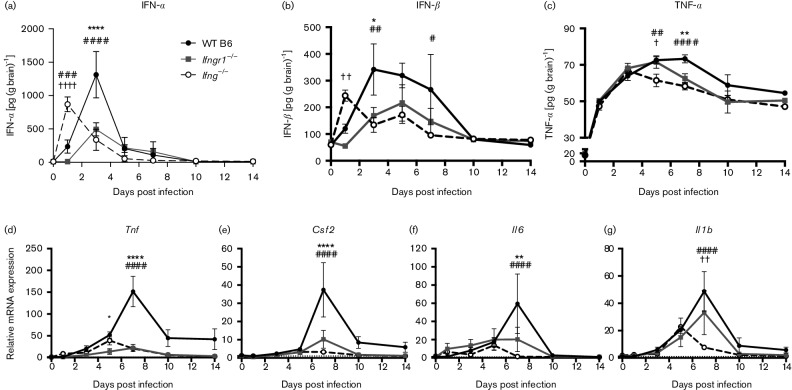
The immune response to CNS infection can be neurotoxic and exacerbate clinical disease (Kimura & Griffin, 2003). To determine how IFN-γ modulated production of cytokines in the CNS, mRNA expression was examined by quantitative PCR and protein production by ELISA and compared to mock-infected controls (Fig. 3). WT B6 mice produced the most IFN-α (Fig. 3a) and IFN-β (Fig. 3b), with levels peaking at 3 DPI. Production was impaired in Ifngr1−/− and Ifng−/− mice, but IFN-α and IFN-β were produced earlier in Ifng−/− mice than the other two strains. Following peak production in WT B6 mice, IFN-α levels quickly decreased, but production of IFN-β declined more slowly (Fig. 3b). IFN-β production was diminished in mice with impaired IFN-γ signalling, with levels only detectable at 1 and 5 DPI in Ifng−/− and Ifngr1−/− brains, respectively. Therefore, IFN-γ signalling affected not only the amounts of IFN-α and IFN-β produced in brains during SINV infection, but also the time during which maximum amounts were made, corresponding with peak infectious virus levels.
Fig. 3.
Cytokine production in the brain during SINV infection. (a–c) IFN-α (a), IFN-β (b) and TNF-α (c) protein levels were measured in the brains of WT B6, Ifngr1−/− and Ifng−/− mice by EIA. Data are presented as mean picograms cytokine per gram brain tissue±sem for three to eight mice per strain per time point. (d–g) Brain mRNA expression of Tnf (d), Csf2 (e), Il6 (f) and il1b (g) was measured by qRT-PCR in WT B6, Ifngr1−/− and Ifng−/− mice and normalized to 0 DPI control mice of each strain. Data are presented as mean relative mRNA expression±sem for three to four mice per strain per time point; dotted line indicates gene expression of 0 DPI brains for each strain to which other time points were normalized; *P<0.05, **P<0.01, ****P<0.0001, WT B6 vs Ifngr1−/−; #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001, WT B6 vs Ifng−/−; †P<0.05, ††P<0.01, ††††P<0.0001, Ifngr1−/− vs Ifng−/−, Tukey’s multiple comparisons test.
At 7 DPI, when WT B6 mice are clearing infectious virus (Fig. 1e) but are still losing weight (Fig. 2b), expression of Tnf (Fig. 3d), Csf2 (Fig. 3e) and Il6 (Fig. 3f) mRNAs was higher in the brains of WT B6 mice than Ifngr1−/− or Ifng−/− mice. Il1b expression was also highest in WT B6 mice (Fig. 3g) and was lower in Ifng−/− mice at 7 DPI than either WT B6 or Ifngr1−/− mice. Some strain-dependent variability was present in baseline expression of cytokine mRNAs, but normalization to uninfected WT B6 mouse brains only affected the significance of the difference between WT B6 and Ifng−/− Il1b expression at 7 DPI (data not shown).
Overall, TNF-α protein production (Fig. 3c) corresponded to Tnf mRNA expression (Fig. 3d), although increases in protein were not as large as the increases in mRNA. TNF-α levels peaked earlier and at lower levels in mice with impaired IFN-γ signalling, with Ifng−/− mice having lower protein levels at 5 DPI and WT B6 mice having higher protein levels at 7 DPI. TNF-α protein production also indirectly followed trends in weight loss during infection (Fig. 2b), with increasing levels coinciding with periods of weight loss. Decreasing TNF-α protein levels corresponded with increases in body weight in WT B6 and Ifngr1−/− mice.
Effect of IFN-γ signalling on SINV-specific antibody production
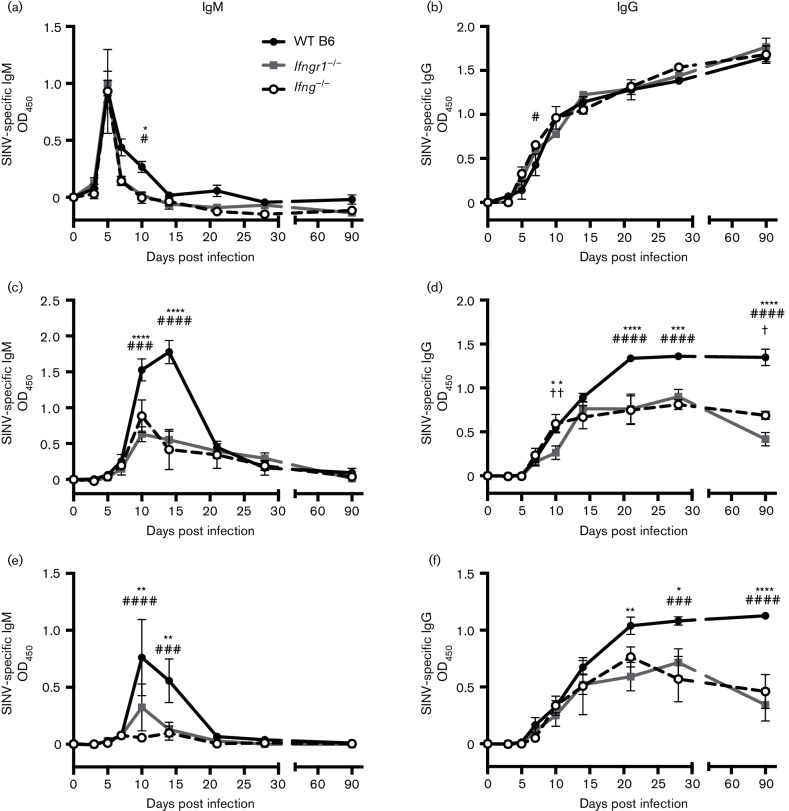
IFN-γ and antibody against the E2 glycoprotein work synergistically to facilitate virus clearance (Burdeinick-Kerr et al., 2007). To determine whether IFN-γ influenced antibody production, SINV-specific IgM and IgG were measured in the sera, brains and spinal cords of WT B6, Ifngr1−/− and Ifng−/− mice. Impaired IFN-γ signalling had little effect on antibody levels in the serum (Fig. 4a, b), with IgM peaking at 5 DPI before dropping below baseline levels by 14 DPI (Fig. 4a) and IgG steadily rising through 90 DPI in all strains (Fig. 4b).
Fig. 4.
Effect of IFN-γ on SINV-specific antibody production. Anti-SINV IgM (a, c, e) and IgG (b, d, f) were measured in the serum (a, b), brains (c, d) and spinal cords (e, f) of WT B6, Ifngr1−/− and Ifng−/− mice by ELISA. Data are presented as mean ODs±sem for three to four mice per strain per time point; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, WT B6 vs Ifngr1−/−; #P<0.05, ###P<0.001, ####P<0.0001, WT B6 vs Ifng−/−; †P<0.05, ††P<0.01, Ifngr1−/− vs Ifng−/−, Tukey’s multiple comparisons test.
In contrast, SINV-specific antibody production in the brain differed over time (Fig. 4c, d). IgM levels were higher in WT B6 brains at 10 and 14 DPI (Fig. 4c). IgG levels increased in the brains of all three strains up to 14 DPI, when levels plateaued in Ifngr1−/− and Ifng−/− mice (Fig. 4d), but continued to increase in WT B6 mice through 21 DPI and remained significantly higher than in Ifngr1−/− and Ifng−/− mice through 90 DPI. These differences in local antibody production among strains were confirmed by an additional independent experiment examining SINV-specific IgG levels in serum and brain collected at late time points.
Similar to brains, SINV-specific antibody production in the spinal cord differed (Fig. 4e, f). IgM levels were higher in WT B6 spinal cords at 10 and 14 DPI (Fig. 4e). IgG levels increased through 90 DPI, with Ifngr1−/− and Ifng−/− mice having significantly lower amounts than WT B6 mice at 21, 28 and 90 DPI (Fig. 4f). Therefore, IFN-γ signalling did not affect the antibody response in the periphery, but substantially affected production of SINV-specific IgM and IgG at the CNS sites of infection.
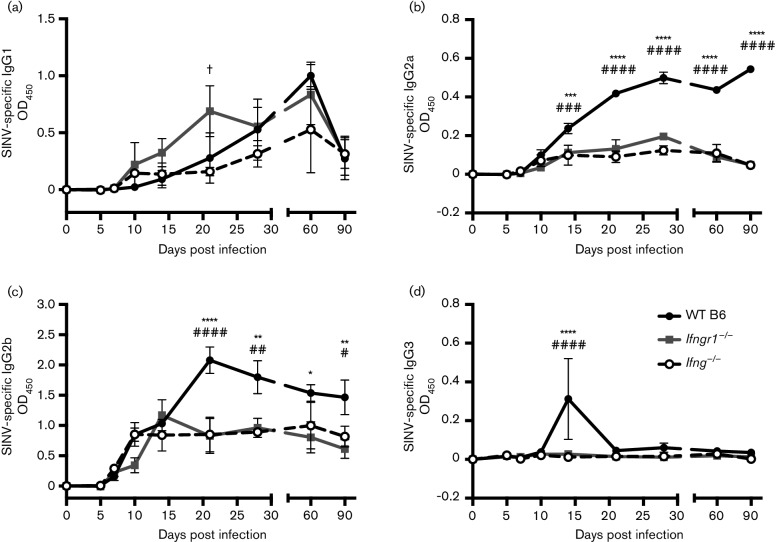
Mice have four IgG subclasses: IgG1, IgG2a, IgG2b and IgG3 (Fahey et al., 1964; Grey et al., 1971; Nussenzweig et al., 1964). Because overall production of SINV-specific IgG in the brain was affected by impaired IFN-γ signaling, we next examined the effects on individual IgG subclasses (Fig. 5). IgG1 levels did not markedly differ between strains (Fig. 5a). IgG2a and IgG2b levels, in contrast, followed a similar pattern to overall IgG, with antibody production continuing to increase in WT B6 mice while plateauing in Ifngr1−/− and Ifng−/− mice (Fig. 5b, c). IgG2a levels were higher in WT B6 mice at 14 DPI (Fig. 5b), and IgG2b production reached higher levels in WT B6 mice at 21 DPI (Fig. 5c). SINV infection only induced production of IgG3 transiently in the brains of WT B6 mice at 14 DPI (Fig. 5d). Therefore, impaired IFN-γ signalling during SINV infection did not affect serum antibody levels or alter IgG subclass switch, but rather impaired IgM, IgG2a and IgG2b antibody production in the CNS.
Fig. 5.
Effect of IFN-γ on production of anti-SINV IgG subclasses in the brain. Anti-SINV IgG1 (a), IgG2a (b), IgG2b (c) and IgG3 (d) were measured in brains of WT B6, Ifngr1−/− and Ifng−/− mouse brains by EIA. Data are presented as mean ODs±sem for three to four mice per strain per time point; *P<0.05, **P<0.01, ****P<0.0001, WT B6 vs Ifngr1−/−; #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001, WT B6 vs Ifng−/−; †P<0.05, Ifngr1−/− vs Ifng−/−, Tukey’s multiple comparisons test.
Effects on recruitment and maturation of B cells in the brain
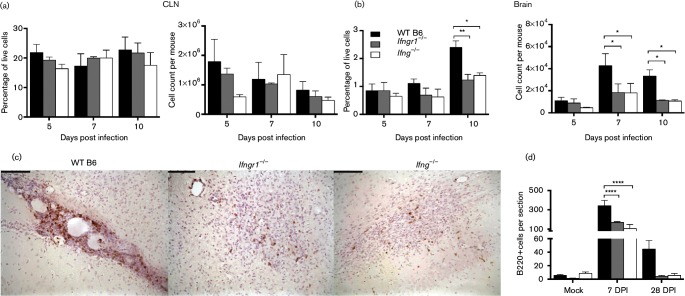
Production of SINV-specific antibody in the nervous system depends on recruitment of B cells into the CNS that proliferate and differentiate into antibody-secreting cells (ASCs) and become resident in the CNS (Metcalf & Griffin, 2011; Metcalf et al., 2013). To determine whether IFN-γ signalling affected production or recruitment of B cells during SINV infection, CD19+ B cells were quantified in the cervical lymph nodes (CLNs) and brain. In CLNs, the draining lymph nodes for the brain, neither the percentage nor absolute number of B cells was significantly affected by impaired IFN-γ signalling (Fig. 6a). In contrast, the percentage of live cells at 10 DPI and absolute number of B cells at 7 and 10 DPI in the CNS were lower in Ifngr1−/− and Ifng−/− mice than WT B6 mice (Fig. 6b). Therefore, while IFN-γ did not affect the induction or proliferation of CD19+ B cells in response to SINV infection in lymphoid tissue, it did affect recruitment of these cells to the CNS.
Fig. 6.
Role of IFN-γ in the proliferation and infiltration of B cells into the CNS during SINV infection. Mononuclear cells were isolated from CLNs (a) and brains (b) of WT B6, Ifngr1−/− and Ifng−/− mice, and the percentage of live cells (left graphs) and absolute number (right graphs) of CD19+ B cells were determined by flow cytometry. Data are represented as the mean±sem of three independent experiments, each consisting of three to seven pooled mice per strain per time point. Formalin-fixed, paraffin-embedded brains from SINV-infected mice at 7 and 28 DPI or mock-infected mice were stained for B220 by IHC to identify infiltrating B cells. (c) Representative photomicrographs of WT B6 (left panel), Ifngr1−/− (centre panel) and Ifng−/− (right panel) mouse brains at 7 DPI are presented (brown staining=B220+ cells; ×200 magnification; bars, 100 µm). (d) B220+ cells were quantified in coronal sections of mock-infected and SINV-infected mouse brains at 7 and 28 DPI. Data are represented as the mean±sem of three to four mice per strain per time point. *P<0.05, **P<0.01, ****P<0.0001, Tukey’s multiple comparisons test.
To further evaluate the infiltration of B cells into the CNS during SINV infection, B220+ cells in the brains of mock-infected and SINV-infected mice were examined by immunohistochemistry (IHC). At 7 DPI in WT B6 mouse brains, B220+ cells were primarily in perivascular cuffs, particularly along the lateral ventricles and choroid plexus; whereas in Ifngr1−/− and Ifng−/− mice, B220+ cells were more commonly distributed throughout the brain parenchyma (Fig. 6c). At 28 DPI, B220+ cells were still present in perivascular cuffs in WT B6 mouse brains, but were only occasionally seen in mice with impaired IFN-γ signalling, similar to mock-infected control mice. Quantification of B220+ cells showed that brains of WT B6 mice had more B220+ cells than Ifngr1−/− or Ifng−/− mice and that B cell numbers decreased in all strains by 28 DPI (Fig. 6d).
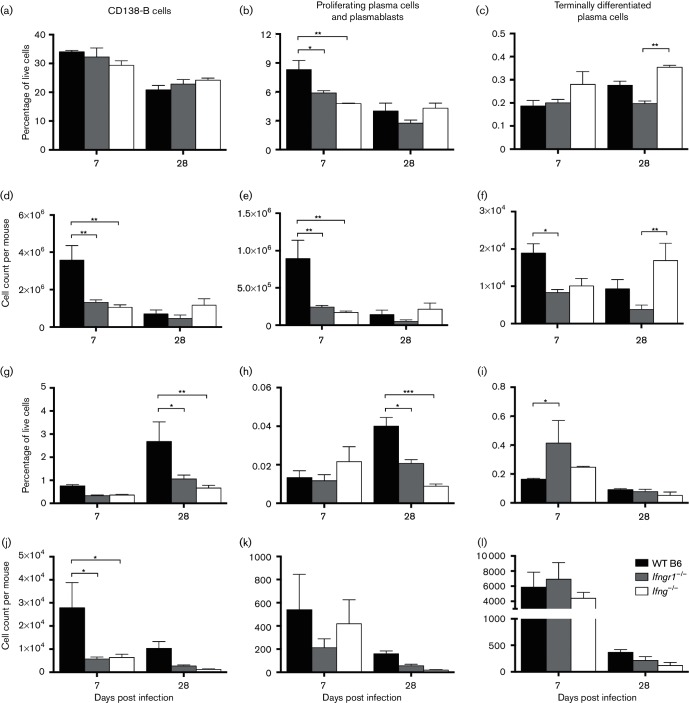
To evaluate the effect of IFN-γ signalling on ASC differentiation, expression of CD38 and CD138 on B cells was examined by flow cytometry (Fig. 7) at 7 and 28 DPI. Activated B cells express CD38 (Vences-Catalán & Santos-Argumedo, 2011), and CD138 is expressed by plasmablasts and plasma cells. While early proliferating plasma cells express CD19, terminally differentiated plasma cells tend to be CD19− (Liu et al., 2012; Medina et al., 2002). Therefore, in evaluating the maturation state of B cells, non-antibody-secreting B cells were defined as CD3−CD19+CD38+CD138− cells, proliferating plasma cells and plasmablasts as CD3−CD19+CD138+ cells and terminally differentiated plasma cells as CD3−CD19−CD138+ cells. Compared to CD138− B cells (Fig. 7a, d), few proliferating plasma cells and plasmablasts (Fig. 7b, e) and even fewer terminally differentiated plasma cells (Fig. 7c, f) were present in the CLNs at 7 and 28 DPI. In the brain, most B cell lineage cells were also CD138− (Fig. 7g), but there were fewer proliferating plasma cells and plasmablasts (Fig. 7h) than terminally differentiated plasma cells (Fig. 7i). While as a percentage of live cells, more CD138− B cells and proliferating plasma cells/plasmablasts were present in the brain at 28 DPI than 7 DPI, in absolute numbers (Fig. 7j–l), there were fewer cells in the brain at 28 DPI. As a percentage of live cells, CD138− B cells (Fig. 7g) and proliferating plasma cells/plasmablasts (Fig. 7h) were lower in mice with impaired IFN-γ signalling at 28 DPI. The absolute number of CD138− B cells at 7 DPI (Fig. 7j) and of all three cell subsets at 28 DPI tended to be lower in Ifngr1−/− and Ifng−/− mice compared to WT B6 mice.
Fig. 7.
Role of IFN-γ in the differentiation of ASCs during SINV infection. Following isolation of mononuclear cells from CLNs (a–f) and brains (g–l) of WT B6, Ifngr1−/− and Ifng−/− mice, non-antibody secreting B cells (CD19+CD38+CD138−), proliferating plasma cells and plasmablasts (CD19+CD138+) and terminally differentiated plasma cells (CD19−CD138+) as a percentage of live cells (a–c, g–i) or as absolute counts (d–f, j–l) were determined by flow cytometry. Data are represented as the mean±sem of two or three independent experiments, each consisting of two to six pooled mice per strain per time point; *P<0.05, **P<0.01, ***P<0.001, Tukey’s multiple comparisons test.
B cell chemokine and growth factor mRNA expression in the brain
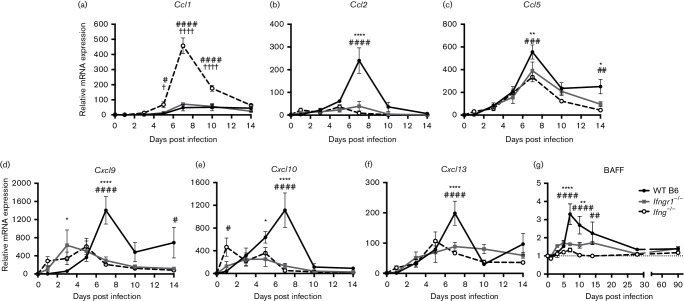
To determine whether the reduced B cell recruitment into the CNS was due to lower chemokine production, brain chemokine mRNA levels were measured. Preliminary data obtained by PCR array on brain mRNAs from 7 DPI identified several differentially expressed chemokine genes that were then selected for further study. Ccl1 expression (Fig. 8a) increased more in Ifng−/− mice compared to both WT B6 and Ifngr1−/− mice at 5, 7 and 10 DPI, but this was almost entirely due to lower baseline Ccl1 mRNA levels in these mice. When compared to the baseline levels for uninfected WT B6 mice, the peak fold increase in expression at 7 DPI was 82 rather than 458 in the brains of Ifng−/−mice. In contrast, for the other chemokine mRNAs examined, WT B6 mice had higher 7 DPI mRNA expression of Ccl2 (Fig. 8b), Ccl5 (Fig. 8c), Cxcl9 (Fig. 8d), Cxcl10 (Fig. 8e) and Cxcl13 (Fig. 8f) compared to Ifngr1−/− and Ifng−/− mice. Baseline Cxcl9 mRNA levels were approximately 10-fold higher in WT B6 mice, so comparison of the expression levels in Ifng−/− and Ifngr1−/− mice to uninfected WT B6 mice increased the differences in expression at 7, 10 and 14 DPI. Baseline expression of other chemokine mRNAs was comparable between mouse strains. These data show that during SINV infection, IFN-γ is important for facilitating chemotaxis of inflammatory cells into the brain.
Fig. 8.
B cell chemokine and growth factor production in the brain during SINV infection. Brain mRNA expression of Ccl1 (a), Ccl2 (b), Ccl5 (c), Cxcl9 (d), Cxcl10 (e), Cxcl13 (f) and BAFF (g) was measured by qRT-PCR in WT B6, Ifngr1−/− and Ifng−/− mice and normalized to 0 DPI control mice of each strain. Data are presented as mean relative mRNA expression±sem for three to four mice per strain per time point; dotted line indicates gene expression of 0 DPI brains for each strain to which other time points were normalized; *P<0.05, **P<0.01, ****P<0.0001, WT B6 vs Ifngr1−/−; #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001, WT B6 vs Ifng−/−; †P<0.05, ††††P<0.0001, Ifngr1−/− vs Ifng−/−, Tukey’s multiple comparisons test.
We also examined mRNA expression of B cell activating factor (BAFF) in WT B6, Ifngr1−/− and Ifng−/− mice through 90 DPI. Compared to WT B6 mice, BAFF mRNA expression was significantly lower in Ifngr1−/− and Ifng−/− mice at 7 and 10 DPI (Fig. 8g) and likely indicates that brains of mice with intact IFN-γ signalling provide a more favourable environment for long-term survival of ASCs.
Discussion
Clearance of infectious virus is accomplished through a cooperative effort between SINV-specific antibody and IFN-γ, and these studies have demonstrated interactions between these two effector mechanisms that affect clinical disease and virus clearance. Onset of clinical disease was earlier in mice with impaired IFN-γ signalling, but these mice had lower CNS levels of IFN-α, IFN-β and TNF-α and lost less weight than WT B6 mice. Chemokine induction, B cell recruitment and antiviral antibody production within the CNS were also reduced in Ifngr1−/− and Ifng−/− mice. Therefore, lack of IFN-γ signalling improved clinical disease by lowering levels of CNS pro-inflammatory cytokines, but impaired the local humoral response to SINV infection by decreasing chemokine and growth factor production important for infiltration and retention of antibody-secreting B cells into the CNS. These findings highlight the multifaceted effects of IFN-γ during virus infection and the trade-offs exerted by inflammatory responses in the CNS.
A number of studies have examined the effects of IFN-γ signalling on neurologic disease using Ifng−/− or Ifngr1−/− mice. Increased mortality is seen in Ifngr1−/− or Ifng−/− mice during CNS infection with viruses such as JHM strain of MHV (JHMV), lymphocytic choriomeningitis virus (LCMV) and Theiler’s murine encephalomyelitis virus (Henrichsen et al., 2005; Parra et al., 1999; Rodriguez et al., 2003; Savarin et al., 2012). In experimental allergic encephalomyelitis and in JHMV-induced autoimmune demyelinating encephalomyelitis, absence of IFN-γ signalling results in more severe demyelinating disease and increased lymphocyte infiltration (Krakowski & Owens, 1996; Pewe et al., 2002; Willenborg et al., 1996). Mice deficient in both IFN-α/β and IFN-γ receptors infected with dengue virus are more likely to develop lethal paralysis and systemic disease than mice deficient in IFN-α/β receptor alone (Prestwood et al., 2012). Furthermore, neutralization of IFN-γ in influenza A virus (IAV)-infected mice results in increased weight loss (Teijaro et al., 2010). These studies all indicate that IFN-γ is protective against pathogen-induced clinical disease.
In our studies, Ifngr1−/− and Ifng−/− mice actually lost less weight than WT B6 mice before recovering, indicating that IFN-γ exacerbated clinical disease during nonfatal alphavirus encephalomyelitis. Ifng−/− mice infected with WNV are protected from developing limbic seizures after administration of N-methyl-d-aspartate or kainic acid compared to WT B6 mice, indicating an important role for IFN-γ in regulating the excitatory seizure pathway (Getts et al., 2007). Glutamate is the major excitatory neurotransmitter and glutamate excitotoxicity linked to local production of TNF-α is implicated in SINV-induced neurologic disease (Carmen et al., 2009; Greene et al., 2008; Nargi-Aizenman & Griffin, 2001; Nargi-Aizenman et al., 2004; Potter et al., 2015).
TNF-α may also be involved in the weight loss that accompanies SINV CNS infection. IFN-γ signalling significantly increased expression of several pro-inflammatory cytokine genes, including Tnf, Csf2, Il1b and Il6. These genes can be expressed by both infiltrating leukocytes and activated resident microglia to contribute to immune cell recruitment and CNS pathological changes (Griffin, 2003). Upregulation of these genes, particularly Tnf, provides a possible explanation for the decreased appetites leading to weight loss seen in WT B6 mice compared to Ifngr1−/− and Ifng−/− mice (Fehr et al., 2015; Feuerstein et al., 1994). TNF-α mediates cachexia and weight loss during chronic diseases such as cancer, and pro-inflammatory cytokines, particularly TNF-α and IL-1β, are implicated in central control of appetite during infection (Langhans, 2000; Langhans & Hrupka, 1999; Plata-Salamán, 1996). In this study, TNF-α protein in the brain was inversely correlated with weight loss in each mouse strain, with decreasing levels of TNF-α corresponding to increasing body weights. Therefore, weight loss induced during alphavirus encephalomyelitis may be mediated through TNF-α-dependent mechanisms.
IFN-γ affects production of the type I IFNs, IFN-α and IFN-β. Levels of IFN-α and IFN-β were lower in the brains of Ifngr1−/− and Ifng−/− mice compared to WT B6 mice at 5 and 3 DPI, respectively. Interestingly, both IFN-α and IFN-β levels were significantly higher in the brains of Ifng−/− mice at 1 DPI compared to WT B6 and Ifngr1−/− mice. This might be driven by higher levels of infectious virus present in the brain at 1 DPI and suggests a role for IFN-γ before T cells infiltrate the site of infection. Differences in virus replication and immune gene expression, as well as clinical disease, between Ifng−/− and Ifngr1−/− mice serve as a reminder that the two strains are not equivalent and suggest that some IFN-γ signalling occurs in Ifngr1−/− mice, either through the mutated receptor or by another mechanism (Kotenko et al., 1995; Lee et al., 2013; Leon et al., 2006; Soh et al., 1994).
Local production of type I IFNs is important for the initial control of virus replication, and mice deficient in type I IFN signalling are highly susceptible to many neurotropic viruses (Burdeinick-Kerr et al., 2007; Byrnes et al., 2000; Grieder & Vogel, 1999; Ireland et al., 2008; Müller et al., 1994). During alphaherpesvirus infection of the peripheral nervous system, IFN-β activates a local antiviral response in axons, whereas IFN-γ induces p-STAT1 accumulation at more distant locations within the neuronal cell body, demonstrating distinct yet cooperative mechanisms by which two different IFNs control virus infection (Song et al., 2016). Type I and II IFNs induce many overlapping ISGs and produce a synergistic antiviral effect against viruses such as LCMV, MHV, IAV and encephalomyocarditis virus (Fuchizaki et al., 2003; Kimura et al., 1994; Levy et al., 1990; Matsumoto et al., 1999; Müller et al., 1994; Stifter et al., 2016). Infection of Ifng−/−mice with pneumonia virus of mice results in reduced expression of types I and III IFNs and 2′,5′-oligoadenylate synthetases, ISGs stimulated by both type I IFNs and IFN-γ (Glineur et al., 2014). Therefore, in addition to the effects directly produced by IFN-γ, the dysregulation of type I IFNs due to altered IFN-γ signalling likely also has a profound effect on the immune response. For example, mRNA expression of Cxcl10, which can be induced by both types I and II IFNs (Cole et al., 1998), was higher in brains of Ifng−/− mice at 1 DPI compared to WT B6 and Ifngr1−/− mice, correlating with increased levels of IFN-α and IFN-β. In contrast, mRNA expression of Cxcl9, which is preferentially induced by IFN-γ (Farber, 1990), was similar among all three strains at 1 DPI. Differences in basal type I IFN levels among mice with impaired IFN-γ signalling can also affect baseline ISG expression. Further examination of the ISGs induced by both type I and II IFNs in WT B6, Ifngr1−/− and Ifng−/− mice during nonfatal alphavirus encephalomyelitis is warranted.
Expression of several chemokine mRNAs was more upregulated in the brains of WT B6 mice than Ifngr1−/− and Ifng−/− mice, including Ccl2, Ccl5, Cxcl9, Cxcl10 and Cxcl13. CCL2, CCL5, CXCL9 and CXCL10 all play important roles in microglial activation and leukocyte migration into the CNS during flavivirus, alphavirus and coronavirus infections and during experimental autoimmune encephalomyelitis (EAE) (Bardina et al., 2015; Hussmann & Fredericksen, 2014; Kulcsar et al., 2015; Lee et al., 2013; Metcalf et al., 2013; Palus et al., 2013; Phares et al., 2013; Quick et al., 2014; Tran et al., 2000; Trujillo et al., 2013). CXCL9 and CXCL10 in particular are important for chemotaxis of B cells into the brain during SINV infection, and CXCL13 is important in non-lymphoid tissue formation of B cell follicles for local antibody production (Metcalf et al., 2013). Furthermore, expression of CXCR3, the receptor for CXCL9 and CXCL10, on ASCs is necessary for proper trafficking to the CNS but not bone marrow during JHMV infection, with CXCR3−/− mice showing delayed viral RNA and infectious virus clearance and sustained clinical disease (Gil-Cruz et al., 2012; Marques et al., 2011). mRNA expression of the chemokine CCL1, which attracts granulocytes, monocytes, NK cells and regulatory T cells and is generally associated with a Th2 response (Colantonio et al., 2002; D'Ambrosio et al., 1998; Miller & Krangel, 1992), was significantly elevated in Ifng−/− mouse brains during SINV infection. While the impact of this difference is likely moderated by lower endogenous levels of Ccl1 in Ifng−/− mice compared to WT B6 and Ifngr1−/− mice, IFN-γ-deficient MRL mice infected with Borna disease virus also have increased brain Ccl1 expression, leading to impaired infiltration of eosinophils, and IFN-γ-deficient SJL/J mice have increased Ccl1 spinal cord expression during induction of EAE (Hausmann et al., 2005; Tran et al., 2000).
Interestingly, the magnitude and duration of IFN-γ and TNF-α protein production did not match that of Ifng or Tnf gene expression in the brain during SINV infection. This dichotomy has previously been seen with IFN-γ in alloactivated T cells treated with anti-CD4 and TNF-α in macrophages infected with IAV (Lehmann et al., 1996). Both of these cytokines are controlled at both the post-transcriptional and post-translational levels through mechanisms such as mRNA stability, decay and localization, controlled access to translational proteins and protein folding and sequestration in the endoplasmic reticulum (Hodge et al., 2002; Raghavan et al., 2002; Sawitzki et al., 2004; Stamou & Kontoyiannis, 2010; Young & Bream, 2007). This phenomenon also applies to ISGs, so examination of both mRNA expression and protein levels is required to fully understand the cytokine and chemokine response to viral infections.
CNS damage observed during fatal alphavirus encephalomyelitis is primarily immune mediated, rather than directly due to the virus; infiltrating T cells in particular are responsible for inducing neuronal cell death (Charles et al., 2001; Kulcsar et al., 2014, 2015; Rowell & Griffin, 1999, 2002). As the quintessential pro-inflammatory cytokine, IFN-γ plays a multifactorial role in modulation of the immune response during infection, and different virus infections elicit diverse T cell responses via IFN-γ signalling. During neuroadapted SINV (NSV) infection, Ifngr1−/− mice have increased CD3+ and CD4+ T cells infiltrating the brain at 7 DPI (Lee et al., 2013). Increased mortality in Ifngr1−/− and Ifng−/− mice during EAE is attributed to IFN-γ-mediated suppression of the CD4+ T cell response, which facilitates EAE-induced pathology (Chu et al., 2000). When mice are infected with a recombinant rabies virus encoding IFN-γ, fewer CD8+ T cells infiltrate the cortex and cerebellum and CD8+ T cells are increased in Ifng−/− mice infected with vaccinia virus (Barkhouse et al., 2015; Remakus & Sigal, 2011). The mechanism by which IFN-γ decreases cytotoxic lymphocyte generation is postulated to be due to limitation of IL-2 production, creating a negative feedback loop (Dalton et al., 1993; Hidalgo et al., 2005). Alternatively, mouse mammary tumour virus infection of Ifngr1−/− mice results in no change in CD8+ T cell, CD4 T cell or B cell numbers in lymph nodes, nor any difference in virus titres (Maillard et al., 1998), and Ifng−/− mice show no difference in CD4+ or CD8+T cells in the spinal cord following infection with Theiler’s murine encephalomyelitis virus (Rodriguez et al., 2003). Although less extensively studied during fatal alphavirus encephalomyelitis, macrophage recruitment in response to JHMV and LCMV infection in the CNS is also affected by IFN-γ signalling (Bergmann et al., 2004; Lin et al., 2009; Pewe et al., 2002; Savarin & Bergmann, 2008). Monocyte/macrophage and T cell responses during nonfatal alphavirus encephalomyelitis deserve further examination.
Persistent viral RNA poses the potential for virus reactivation and relapse of clinical disease. Because the local presence of long-term ASCs coincides with persistent viral RNA during SINV infection, antibody likely plays an important role in the prevention of virus reactivation (Metcalf & Griffin, 2011; Tyor et al., 1992). Studies examining viral RNA levels in Ifngr1−/− and Ifng−/− mice are warranted to better understand the role of IFN-γ signalling in clearance of viral RNA and the effect of local antibody presence on virus persistence.
BAFF is an IFN-γ-induced TNF family member protein that is essential for plasma cell maintenance in the bone marrow (Benson et al., 2008) and is produced by astrocytes in the brain (Krumbholz et al., 2005; Thangarajh et al., 2007). Although both BAFF and IFN-γ-independent proliferation-inducing ligand are upregulated during some CNS infections (Phares et al., 2011; Tschen et al., 2006), only BAFF mRNA is increased in the brain during alphavirus infection, along with BAFF receptor expression on B cells infiltrating the brain (Metcalf et al., 2013). Expression of BAFF mRNA was lower in the absence of IFN-γ signalling, suggesting a less supportive environment for ASC differentiation and survival than in WT B6 animals.
Antibody is necessary for successful clearance of several viruses, including respiratory syncytial virus, norovirus, haemagglutinating encephalomyelitis virus, mouse polyoma virus, JHMV and rabies virus (Chachu et al., 2008; Dietzschold, 1993; Hirano et al., 2006; Hooper et al., 2009; Prince et al., 1990; Ramakrishna et al., 2003; Szomolanyi-Tsuda & Welsh, 1996). Previous mouse studies have shown that clearance of infectious SINV is accomplished cooperatively between anti-SINV antibody and IFN-γ. Passive transfer experiments have shown that small amounts of antibody are sufficient for virus clearance (Levine & Griffin, 1992) and antibody, particularly IgM, is most efficiently delivered by ASCs in the CNS. As previously shown, production of SINV-specific IgM and IgG subclasses in serum did not significantly differ among WT B6, Ifngr1−/− and Ifng−/− mice (Burdeinick-Kerr et al., 2007). However, SINV-specific IgM, IgG, IgG2a and IgG2b in the CNS were decreased in Ifngr1−/− and Ifng−/− mice compared to WT B6 mice. Effects on subclass distribution are consistent with observations that Th1 cells producing IFN-γ induce IgG subclass switching towards IgG2a, while Th2 cells producing IL-4 and IL-13 promote subclass switching towards IgG1 and away from IgG2a (Snapper & Paul, 1987; Stevens et al., 1988). IgG1 levels were significantly higher in the brains of Ifngr1−/− mice compared to Ifng−/− mice at 21 DPI, and while both Ifngr1−/− and Ifng−/− mice were previously found to have higher serum IgG1 levels compared to WT B6 mice (Burdeinick-Kerr et al., 2007), exploration of the T cell response during SINV TE infection and its effect on the anti-SINV antibody response in the context of IFN-γ signalling would help further explain our findings.
Because the numbers of B cells were lower in the brains of mice with impaired IFN-γ signalling, the effects of IFN-γ signalling on antibody are likely due to differences in local B cell production of anti-SINV antibody during infection. At 28 DPI, when SINV-specific antibody levels were significantly lower in Ifngr1−/− and Ifng−/− mice, WT B6 mice had more B220+ cells as evaluated by IHC and more CD138− B cells and proliferating plasma cells/plasmablasts as evaluated by flow cytometry residing in the brain compared to Ifngr1−/− and Ifng−/− mice. Type I IFN also enhances primary antibody production and influences IgG isotype switching, making it an important adjuvant in inducing immunological memory (Coro et al., 2006; Finkelman et al., 1991; Le Bon et al., 2001, 2006; Swanson et al., 2010). The reduced IFN-α and IFN-β production in the brains of Ifngr1−/− and Ifng−/− mice adds another mechanism by which IFN-γ signalling can affect the humoral immune response to SINV infection.
In our studies, ASCs, designated as CD19+CD138+ (proliferating plasma cells and plasmablasts) or CD19−CD138+ (terminally differentiated plasma cells), decreased in number in the brains of SINV-infected mice from 7 to 28 DPI, despite progressively increasing levels of IgG in the CNS. These results, plus previous studies that showed increasing numbers of SINV-specific ASCs (Metcalf & Griffin, 2011), are indicative of continued selection for ASCs producing relevant virus-specific antibody during maturation of the local CNS immune response.
Expression of chemokine mRNAs Cxcl9, Cxcl10 and Cxcl13, which are all important for inducing migration and promoting survival of ASCs during neurotropic viral infections (Metcalf et al., 2013; Phares et al., 2016), were reduced in Ifngr1−/− and Ifng−/− mouse brains compared to WT B6 mice. Therefore, IFN-γ-induced expression of B cell-associated chemokines affects migration and maintenance of ASCs in the CNS, and thus local production of antibody. The promotion of local antibody production by IFN-γ provides a mechanism by which IFN-γ and SINV-specific antibody cooperatively clear infectious SINV from the CNS and highlights the multifaceted role IFN-γ plays in modulating the immune response during alphavirus encephalomyelitis.
Methods
Virus infection of mice.
Stocks of the TE strain of SINV were grown and assayed in BHK-21 cells (Lustig et al., 1988). Four to six week old C57BL/6 mice (WT B6), mice deficient in IFN-γ receptor 1 (Ifngr1−/−, strain B6.129S7-Ifngtm1Ts/J) and mice deficient in IFN-γ (Ifng−/−, strain B6.129S7-Ifngr1tm1Agt/J) were acquired from the Jackson Laboratory and bred in-house. Mice were intracranially inoculated with 103 p.f.u. of SINV TE diluted in 20 µl PBS or with 20 µl PBS vehicle under light isoflurane anaesthesia. To collect tissues, mice were euthanized by an overdose of isoflurane, and blood, CLNs, brains and spinal cords were collected. Equal numbers of male and female mice were used whenever possible. The Johns Hopkins University Institutional Animal Care and Use Committee approved the protocols for all studies performed.
Clinical evaluation of mice.
Mice were weighed daily and evaluated for signs of encephalomyelitis, characterized by kyphosis, abnormal gait and paresis. For food intake studies, mice were housed in groups of two or three and total feed weight was recorded daily. The amount of feed consumed per day per mouse was calculated and normalized to that of mock-infected controls. Body temperature was measured rectally using a Physitemp Thermalert TH-5 with a RET-3 rectal probe. Investigators were blinded to the strain and infection status of mice, and evaluations were conducted at the same time each day.
Quantification of infectious virus.
Left brain hemispheres were placed in Lysing Matrix A tubes (MP Biomedicals) and 20 % w/v homogenates made using ice-cold PBS. Whole spinal cords were handled similarly to make 10 % w/v homogenates. Tissues were dissociated using a FastPrep-24 homogenizer (MP Biomedicals) at 6 m s−1 for 40 s and clarified by centrifugation for 15 min at 13 200 r.p.m. at 4 °C. Supernatant fluids were collected and stored at −80 °C. Infectious virus was quantified by plaque assay on BHK cells. For samples in which plaques could not be detected, a titre value of half of the limit of detection for that assay was assigned.
mRNA measurement by real-time PCR.
Right brain hemispheres and whole spinal cords from at least three individual mice per strain per time point were homogenized in Lysing Matrix E tubes as above. A Qiagen RNeasy Lipid Tissue Mini kit (Valencia) was used to isolate RNA, and cDNA was synthesized using a High Capacity cDNA Reverse Transcription kit with random primers (Life Technologies). Preliminary gene expression data in mouse brains at 7 DPI were obtained using the Mouse Innate & Adaptive Immune Responses RT² Profiler PCR Array (SABiosciences) according to manufacturer’s instructions. Quantitative real-time PCR examining selected individual genes was performed using TaqMan Universal PCR Master Mix (Roche) on a 7500 Fast Real-Time PCR System (Applied Biosystems) and commercially available TaqMan gene expression assays (Integrated DNA Technologies or Applied Biosystems). Results were analysed using Sequence Detection software, version 1.4 (Applied Biosystems). Relative quantification was performed by the ΔΔCt method using endogenous mouse glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA for normalization.
Protein quantification by enzyme immunoassay.
IFN-α, IFN-β, IFN-γ and TNF-α levels in 20 % w/v brain homogenates and 10 % w/v spinal cord homogenates were quantified by EIA. VeriKine Mouse Interferon Alpha and Interferon Beta ELISA kits (PBL Interferon Source) were used to measure type I IFN. IFN-γ and TNF-α were measured using the mouse IFN-γ and TNF-α Ready-SET-Go! kits (eBioscience). Brain homogenates tested were diluted 1 : 2 for type I IFNs, 1 : 4 for IFN-γ and undiluted for TNF-α. Spinal cord homogenates were diluted 1 : 2 for the IFN-γ EIA. Assays were performed according to manufacturers’ instructions, and data are presented as picograms per gram tissue. The IFN-α and TNF-α cytokine levels were too low to be interpolated by the standard curve for a few samples. In these cases, samples were assigned a value of 10 and 15 pg g−1, respectively, equal to approximately half of the lowest interpolated value.
Anti-SINV antibody was measured in sera, brain homogenates and spinal cord homogenates using an in-house EIA. MaxiSorp 96-well plates (Thermo Scientific Nunc) were coated with 106 p.f.u. PEG-purified SINV in coating buffer (50 mM NaHCO3, pH 9.6) and incubated overnight at 4 °C. Plates were blocked with PBS–0.05 % Tween 20 + 10 % FBS (PBST-10 % FBS) for 2 h at 37 °C. Samples diluted in PBST-10 % FBS (1 : 100 for serum, 1 : 4 for brain homogenates and 1 : 2 for spinal cord homogenates) were added and incubated overnight at 4 °C. Washed wells were incubated with 1 : 1000 HRP-conjugated goat anti-mouse IgG2a, IgG2b or IgG3 or 1 : 2000 HRP-conjugated goat anti-mouse IgM, IgG or IgG1 (Southern Biotech) for 2 h at room temperature, and plates were developed with a BD OptEIA TMB Substrate Reagent kit using 2 M H2SO4 as stop solution. Plates were read at 450 nm, and OD values for mock-infected mice of each strain were subtracted from the OD values of infected mice.
Mononuclear cell isolation.
Single cell suspensions were prepared from CLNs and brains pooled from two to seven mice per strain per time point. CLNs were dissociated in RPMI + 1 % FBS using gentleMACS C tubes and Dissociator (Miltenyi Biotec) and filtered through a 70 µm pore size cell strainer. Cells were pelleted by centrifugation and red blood cells were lysed using ammonium chloride (Sigma-Aldrich). Remaining cells were filtered through another 70 µm strainer, pelleted by centrifugation and resuspended in PBS + 2 mM EDTA (PE buffer) for counting. Brains were placed in RPMI containing 1 % FBS, 1 mg ml−1 collagenase D (Roche) or 0.5 mg ml−1 collagenase IV (Worthington Biochemical) and 0.1 mg ml−1 DNase I (Roche) and dissociated in C tubes using a gentleMACS Dissociator. Tissues were incubated for 30 min at 37 °C with periodic agitation and dissociated cells were filtered through a 70 µm pore size cell strainer and pelleted by centrifugation. Cell pellets were resuspended in 30 % supplemented Percoll [9 : 1 Percoll (GE Healthcare): 10× salt solution of 80 g NaCl, 3 g KCl, 0.73 g Na2HPO4, 0.2 g KH2PO4 and 20 g glucose in 1 l dH2O] in RPMI in a 15 ml conical tube and underlaid with 70 % supplemented Percoll in RPMI. Tubes were centrifuged at 850 g at 4 °C for 30 min with slow braking and cells were collected from the 30/70 % interface. Cells were washed and resuspended in PE buffer for counting. Live mononuclear cells were identified by trypan blue exclusion.
Flow cytometry.
In the wells of a 96-well round bottom plate, 106 live cells were placed. A violet LIVE/DEAD fixable dead cell stain (Life Technologies) diluted in PE buffer was added followed by incubation with anti-mouse CD16/CD32 (BD Pharmingen) diluted in PE buffer to block Fc receptors. Cells were stained with mAbs against CD45 (clone 30-F11), CD3 (clone 17A2), CD19 (clone 1D3), CD38 (clone 90) and CD138 (clone 281-2) from eBioscience or BD Pharmingen diluted in PBS + 2 mM EDTA + 0.5 % BSA (FACS buffer) for 30 min on ice. Cells were run on a BD FACSCanto II cytometer using BD FACSDiva software, version 8, and analyses used FlowJo software, version 8. All flow cytometry data are averaged representations of two or three independent experiments.
Immunohistochemistry.
Following euthanasia, mice were perfused with PBS followed by 4 % paraformaldehyde, and brains were removed. Brains were cut into three coronal sections using an Adult Mouse Brain Slicer (Zivic Instruments), fixed overnight in 4 % paraformaldehyde at 4 °C and embedded in paraffin. Ten micrometre sections were deparaffinized, hydrated and boiled in 0.01 M sodium citrate for antigen retrieval. Endogenous peroxidases were quenched with 3 % H2O2 in methanol, and slides were blocked in 10 % normal goat serum (NGS). Slides were incubated with rat anti-CD45R/B220 (ab64100, diluted 1 : 200 in PBS + 5 % NGS + 0.04 % Triton-X; Abcam) for 1 h, biotinylated anti-rat IgG (diluted 1 : 300 in PBS + 5 % NGS + 0.04 % Triton-X; Vector Labs) for 30 min, and avidin–biotin complex (VECTASTAIN Elite ABC kit; Vector Labs) for 40 min and were developed in 3,3′-diaminobenzidine (Vector Labs) for 8 min. Tissues were counterstained in haematoxylin, dehydrated and mounted with Permount (Fisher Scientific). After coding the slides to blind the quantifier, B220+ cells, as determined by brown cellular staining, were counted in the centre coronal section (containing hippocampus, midbrain and cortex) of each brain. Brains collected from uninfected, PBS-inoculated mice at 3 and 5 DPI and from SINV-infected mice at 7 and 28 DPI were evaluated.
Statistics.
Statistical analyses were performed using GraphPad Prism 6 software. Time-course studies were analysed by two-way ANOVA with Bonferroni’s or Tukey’s multiple comparison post-test for two group and three group comparisons, respectively. Three outliers were identified in the IFN-γ protein brain data by the ROUT method with a Q=1 % and removed from analyses (one data point each for WT B6 at 3 and 7 DPI and for Ifngr1−/− at 21 DPI). One outlier was identified at 11 DPI in the Ifngr1−/− group of the feed intake study by the same method and was removed from analysis. A P-value of <0.05 was considered significant.
Acknowledgements
This work was supported by research grants R01 NS038932 (D. E. G.) and T32 OD011089 (V. K. B.) from the USA National Institutes of Health. We thank Drs Kimberly Schultz and Kirsten Kulcsar for helpful discussions and Elizabeth Troisi, Jane Xie and Rebecca Glowinski for technical assistance, all of the Johns Hopkins Bloomberg School of Public Health W. Harry Feinstone Department of Molecular Microbiology and Immunology. We additionally express our appreciation to Sabra Klein of the Johns Hopkins Bloomberg School of Public Health W. Harry Feinstone Department of Molecular Microbiology and Immunology for the use of the thermometer to measure body temperature.
References
- Bardina S. V., Michlmayr D., Hoffman K. W., Obara C. J., Sum J., Charo I. F., Lu W., Pletnev A. G., Lim J. K.(2015). Differential roles of chemokines CCL2 and CCL7 in monocytosis and leukocyte migration during West Nile virus infection. J Immunol 1954306–4318. 10.4049/jimmunol.1500352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhouse D. A., Garcia S. A., Bongiorno E. K., Lebrun A., Faber M., Hooper D. C.(2015). Expression of interferon gamma by a recombinant rabies virus strongly attenuates the pathogenicity of the virus via induction of type I interferon. J Virol 89312–322. 10.1128/JVI.01572-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. J., Dillon S. R., Castigli E., Geha R. S., Xu S., Lam K. P., Noelle R. J.(2008). Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol 1803655–3659. 10.4049/jimmunol.180.6.3655 [DOI] [PubMed] [Google Scholar]
- Bergmann C. C., Parra B., Hinton D. R., Ramakrishna C., Dowdell K. C., Stohlman S. A.(2004). Perforin and gamma interferon-mediated control of coronavirus central nervous system infection by CD8 T cells in the absence of CD4 T cells. J Virol 781739–1750. 10.1128/JVI.78.4.1739-1750.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder G. K., Griffin D. E.(2001). Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293303–306. 10.1126/science.1059742 [DOI] [PubMed] [Google Scholar]
- Bruyn H. B., Lennette E. H.(1953). Western equine encephalitis in infants; a report on three cases with sequelae. Calif Med 79362–366. [PMC free article] [PubMed] [Google Scholar]
- Burdeinick-Kerr R., Wind J., Griffin D. E.(2007). Synergistic roles of antibody and interferon in noncytolytic clearance of Sindbis virus from different regions of the central nervous system. J Virol 815628–5636. 10.1128/JVI.01152-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdeinick-Kerr R., Govindarajan D., Griffin D. E.(2009). Noncytolytic clearance of Sindbis virus infection from neurons by gamma interferon is dependent on Jak/STAT signaling. J Virol 833429–3435. 10.1128/JVI.02381-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes A. P., Durbin J. E., Griffin D. E.(2000). Control of Sindbis virus infection by antibody in interferon-deficient mice. J Virol 743905–3908. 10.1128/JVI.74.8.3905-3908.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmen J., Rothstein J. D., Kerr D. A.(2009). Tumor necrosis factor-α modulates glutamate transport in the CNS and is a critical determinant of outcome from viral encephalomyelitis. Brain Res 1263143–154. 10.1016/j.brainres.2009.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachu K. A., Strong D. W., LoBue A. D., Wobus C. E., Baric R. S., Virgin H. W.(2008). Antibody is critical for the clearance of murine norovirus infection. J Virol 826610–6617. 10.1128/JVI.00141-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P. C., Trgovcich J., Davis N. L., Johnston R. E.(2001). Immunopathogenesis and immune modulation of Venezuelan equine encephalitis virus-induced disease in the mouse. Virology 284190–202. 10.1006/viro.2001.0878 [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Wittmer S., Dalton D. K.(2000). Failure to suppress the expansion of the activated CD4 T cell population in interferon γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med 187123–128. 10.1084/jem.192.1.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantonio L., Iellem A., Sinigaglia F., D'Ambrosio D.(2002). Skin-homing CLA+ T cells and regulatory CD25+ T cells represent major subsets of human peripheral blood memory T cells migrating in response to CCL1/I-309. Eur J Immunol 323506–3514. [DOI] [PubMed] [Google Scholar]
- Cole K. E., Strick C. A., Paradis T. J., Ogborne K. T., Loetscher M., Gladue R. P., Lin W., Boyd J. G., Moser B., et al. (1998). Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med 1872009–2021. 10.1084/jem.187.12.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coro E. S., Chang W. L., Baumgarth N.(2006). Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol 1764343–4351. 10.4049/jimmunol.176.7.4343 [DOI] [PubMed] [Google Scholar]
- D'Ambrosio D., Iellem A., Bonecchi R., Mazzeo D., Sozzani S., Mantovani A., Sinigaglia F.(1998). Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol 1615111–5115. [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A.(1993). Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 2591739–1742. 10.1126/science.8456300 [DOI] [PubMed] [Google Scholar]
- Dietzschold B.(1993). Antibody-mediated clearance of viruses from the mammalian central nervous system. Trends Microbiol 163–66. 10.1016/0966-842X(93)90035-P [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest M. P., Goolishian H. A., Calverley J. R., Hayes R. O., Hill H. R.(1971). Neurologic, intellectual, and psychologic sequelae following western encephalitis. A follow-up study of 35 cases. Neurology 21969–974. 10.1212/WNL.21.9.969 [DOI] [PubMed] [Google Scholar]
- Fahey J. L., Wunderlich J., Mishell R.(1964). The immunoglobulins of mice II. Two subclasses of mouse 7S γ2-globulins: γ2a- and γ2b-globulins. J Exp Med 120243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M.(1990). A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci U S A 875238–5242. 10.1073/pnas.87.14.5238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D.(1993). The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol 11571–611. 10.1146/annurev.iy.11.040193.003035 [DOI] [PubMed] [Google Scholar]
- Fehr A. R., Athmer J., Channappanavar R., Phillips J. M., Meyerholz D. K., Perlman S.(2015). The nsp3 macrodomain promotes virulence in mice with coronavirus-induced encephalitis. J Virol 891523–1536. 10.1128/JVI.02596-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein G. Z., Liu T., Barone F. C.(1994). Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev 6341–360. [PubMed] [Google Scholar]
- Finkelman F. D., Svetic A., Gresser I., Snapper C., Holmes J., Trotta P. P., Katona I. M., Gause W. C.(1991). Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J Exp Med 1741179–1188. 10.1084/jem.174.5.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley K. H., Longshore W. A., Palmer R. J., Cook R. E., Riggs N.(1955). Western equine and St. Louis encephalitis; preliminary report of a clinical follow-up study in California. Neurology 5223–235. 10.1212/WNL.5.4.233 [DOI] [PubMed] [Google Scholar]
- Frolov I., Akhrymuk M., Akhrymuk I., Atasheva S., Frolova E. I.(2012). Early events in alphavirus replication determine the outcome of infection. J Virol 865055–5066. 10.1128/JVI.07223-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchizaki U., Kaneko S., Nakamoto Y., Sugiyama Y., Imagawa K., Kikuchi M., Kobayashi K.(2003). Synergistic antiviral effect of a combination of mouse interferon-α and interferon-γ on mouse hepatitis virus. J Med Virol 69188–194. 10.1002/jmv.10286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger K. D., Gurushanthaiah D., Howes E. L., Lewandowski G. A., Reed J. C., Bloom F. E., Sarvetnick N. E.(1995). Cytokine-mediated survival from lethal herpes simplex virus infection: role of programmed neuronal death. Proc Natl Acad Sci U S A 923411–3415. 10.1073/pnas.92.8.3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts D. R., Matsumoto I., Müller M., Getts M. T., Radford J., Shrestha B., Campbell I. L., King N. J.(2007). Role of IFN-γ in an experimental murine model of West Nile virus-induced seizures. J Neurochem 1031019–1030. 10.1111/j.1471-4159.2007.04798.x [DOI] [PubMed] [Google Scholar]
- Gil-Cruz C., Perez-Shibayama C., Firner S., Waisman A., Bechmann I., Thiel V., Cervantes-Barragan L., Ludewig B.(2012). T helper cell- and CD40-dependent germline IgM prevents chronic virus-induced demyelinating disease. Proc Natl Acad Sci U S A 1091233–1238. 10.1073/pnas.1115154109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glineur S. F., Bowen A. B., Percopo C. M., Garcia-Crespo K. E., Dyer K. D., Ochkur S. I., Lee N. A., Lee J. J., Domachowske J. B., Rosenberg H. F.(2014). Sustained inflammation and differential expression of interferons type I and III in PVM-infected interferon-gamma (IFNγ) gene-deleted mice. Virology 468-470140–149. 10.1016/j.virol.2014.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene I. P., Lee E. Y., Prow N., Ngwang B., Griffin D. E.(2008). Protection from fatal viral encephalomyelitis: AMPA receptor antagonists have a direct effect on the inflammatory response to infection. Proc Natl Acad Sci U S A 1053575–3580. 10.1073/pnas.0712390105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Hirst J. W., Cohn M.(1971). A new mouse immunoglobulin: IgG3. J Exp Med 133289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder F. B., Vogel S. N.(1999). Role of interferon and interferon regulatory factors in early protection against Venezuelan equine encephalitis virus infection. Virology 257106–118. 10.1006/viro.1999.9662 [DOI] [PubMed] [Google Scholar]
- Griffin D. E.(2003). Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol 3493–502. 10.1038/nri1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E.(2010a). Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunol Res 47123–133. 10.1007/s12026-009-8143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E.(2010b). Emergence and re-emergence of viral diseases of the central nervous system. Prog Neurobiol 9195–101. 10.1016/j.pneurobio.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E.(2013). Alphaviruses. Fields Virology, 6th edn 652–686. Edited by Knipe D. M., Howley P. M.Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Gubler D. J.(2002). The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res 33330–342. 10.1016/S0188-4409(02)00378-8 [DOI] [PubMed] [Google Scholar]
- Hallensleben W., Staeheli P.(1999). Inhibition of Borna disease virus multiplication by interferon: cell line differences in susceptibility. Arch Virol 1441209–1216. 10.1007/s007050050580 [DOI] [PubMed] [Google Scholar]
- Hausmann J., Pagenstecher A., Baur K., Richter K., Rziha H. J., Staeheli P.(2005). CD8 T cells require γ interferon to clear Borna disease virus from the brain and prevent immune system-mediated neuronal damage. J Virol 7913509–13518. 10.1128/JVI.79.21.13509-13518.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichsen P., Bartholdy C., Christensen J. P., Thomsen A. R.(2005). Impaired virus control and severe CD8+ T-cell-mediated immunopathology in chimeric mice deficient in gamma interferon receptor expression on both parenchymal and hematopoietic cells. J Virol 7910073–10076. 10.1128/JVI.79.15.10073-10076.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo L. G., Urmson J., Halloran P. F.(2005). IFN-γ decreases CTL generation by limiting IL-2 production: a feedback loop controlling effector cell production. Am J Transplant 5651–661. 10.1111/j.1600-6143.2005.00761.x [DOI] [PubMed] [Google Scholar]
- Hirano N., Taira H., Sato S., Hashikawa T., Tohyama K.(2006). Antibody-mediated virus clearance from neurons of rats infected with hemagglutinating encephalomyelitis virus. Adv Exp Med Biol 581391–394. 10.1007/978-0-387-33012-9_69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge D. L., Martinez A., Julias J. G., Taylor L. S., Young H. A.(2002). Regulation of nuclear gamma interferon gene expression by interleukin 12 (IL-12) and IL-2 represents a novel form of posttranscriptional control. Mol Cell Biol 221742–1753. 10.1128/MCB.22.6.1742-1753.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Phares T. W., Fabis M. J., Roy A.(2009). The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl Trop Dis 3e535. 10.1371/journal.pntd.0000535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussmann K. L., Fredericksen B. L.(2014). Differential induction of CCL5 by pathogenic and non-pathogenic strains of West Nile virus in brain endothelial cells and astrocytes. J Gen Virol 95862–867. 10.1099/vir.0.060558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland D. D., Stohlman S. A., Hinton D. R., Atkinson R., Bergmann C. C.(2008). Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells. J Virol 82300–310. 10.1128/JVI.01794-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Lundkvist G., Dons L., Kristensson K., Rottenberg M. E.(2004). Interferon-γ mediates neuronal killing of intracellular bacteria. Scand J Immunol 60437–448. 10.1111/j.0300-9475.2004.01500.x [DOI] [PubMed] [Google Scholar]
- Kimura T., Nakayama K., Penninger J., Kitagawa M., Harada H., Matsuyama T., Tanaka N., Kamijo R., Vilcek J., Mak T. W.(1994). Involvement of the IRF-1 transcription factor in antiviral responses to interferons. Science 2641921–1924. 10.1126/science.8009222 [DOI] [PubMed] [Google Scholar]
- Kimura T., Griffin D. E.(2003). Extensive immune-mediated hippocampal damage in mice surviving infection with neuroadapted Sindbis virus. Virology 31128–39. [DOI] [PubMed] [Google Scholar]
- Klein R. S., Diamond M. S.(2008). Immunological headgear: antiviral immune responses protect against neuroinvasive West Nile virus. Trends Mol Med 14286–294. 10.1016/j.molmed.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Kotenko S. V., Izotova L. S., Pollack B. P., Mariano T. M., Donnelly R. J., Muthukumaran G., Cook J. R., Garotta G., Silvennoinen O., Ihle J. N.(1995). Interaction between the components of the interferon gamma receptor complex. J Biol Chem 27020915–20921. 10.1074/jbc.270.36.20915 [DOI] [PubMed] [Google Scholar]
- Krakowski M., Owens T.(1996). Interferon-γ confers resistance to experimental allergic encephalomyelitis. Eur J Immunol 261641–1646. 10.1002/eji.1830260735 [DOI] [PubMed] [Google Scholar]
- Krumbholz M., Theil D., Derfuss T., Rosenwald A., Schrader F., Monoranu C. M., Kalled S. L., Hess D. M., Serafini B., et al. (2005). BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med 201195–200. 10.1084/jem.20041674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcsar K. A., Baxter V. K., Greene I. P., Griffin D. E.(2014). Interleukin 10 modulation of pathogenic Th17 cells during fatal alphavirus encephalomyelitis. Proc Natl Acad Sci U S A 11116053–16058. 10.1073/pnas.1418966111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcsar K. A., Baxter V. K., Abraham R., Nelson A., Griffin D. E.(2015). Distinct immune responses in resistant and susceptible strains of mice during neurovirulent alphavirus encephalomyelitis. J Virol 898280–8291. 10.1128/JVI.00173-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L., Scott T. W., Gubler D. J.(2010). Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis 4e646. 10.1371/journal.pntd.0000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans W., Hrupka B.(1999). Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides 33415–424. 10.1054/npep.1999.0048 [DOI] [PubMed] [Google Scholar]
- Langhans W.(2000). Anorexia of infection: current prospects. Nutrition 16996–1005. 10.1016/S0899-9007(00)00421-4 [DOI] [PubMed] [Google Scholar]
- Le Bon A., Schiavoni G., D'Agostino G., Gresser I., Belardelli F., Tough D. F.(2001). Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14461–470. 10.1016/S1074-7613(01)00126-1 [DOI] [PubMed] [Google Scholar]
- Le Bon A., Thompson C., Kamphuis E., Durand V., Rossmann C., Kalinke U., Tough D. F.(2006). Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol 1762074–2078. 10.4049/jimmunol.176.4.2074 [DOI] [PubMed] [Google Scholar]
- Lee E.-Y., Schultz K. L. W., Griffin D. E.(2013). Mice deficient in interferon-gamma or interferon-gamma receptor 1 have distinct inflammatory responses to acute viral encephalomyelitis. PLoS One 8 e76412 10.1371/journal.pone.0076412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C., Sprenger H., Nain M., Bacher M., Gemsa D.(1996). Infection of macrophages by influenza A virus: characteristics of tumour necrosis factor-α (TNFα) gene expression. Res Virol 147123–130. 10.1016/0923-2516(96)80226-3 [DOI] [PubMed] [Google Scholar]
- Leon C., Nandan D., Lopez M., Moeenrezakhanlou A., Reiner N. E.(2006). Annexin V associates with the IFN-γ receptor and regulates IFN-γ signaling. J Immunol 1765934–5942. 10.4049/jimmunol.176.10.5934 [DOI] [PubMed] [Google Scholar]
- Levine B., Hardwick J. M., Trapp B. D., Crawford T. O., Bollinger R. C., Griffin D. E.(1991). Antibody-mediated clearance of alphavirus infection from neurons. Science 254856–860. 10.1126/science.1658936 [DOI] [PubMed] [Google Scholar]
- Levine B., Griffin D. E.(1992). Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J Virol 666429–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Lew D. J., Decker T., Kessler D. S., Darnell J. E.(1990). Synergistic interaction between interferon-alpha and interferon-gamma through induced synthesis of one subunit of the transcription factor ISGF3. EMBO J 91105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. A., Tripathi P. K., Sholl A., Jordan M. B., Hildeman D. A.(2009). Gamma interferon signaling in macrophage lineage cells regulates central nervous system inflammation and chemokine production. J Virol 838604–8615. 10.1128/JVI.02477-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Lin P., Hu Y., Zhou Y., Tang G., Powers L., Medeiros L. J., Jorgensen J. L., Wang S. A.(2012). Immunophenotypic heterogeneity of normal plasma cells: comparison with minimal residual plasma cell myeloma. J Clin Pathol 65823–829. 10.1136/jclinpath-2012-200881 [DOI] [PubMed] [Google Scholar]
- Lustig S., Jackson A. C., Hahn C. S., Griffin D. E., Strauss E. G., Strauss J. H.(1988). Molecular basis of Sindbis virus neurovirulence in mice. J Virol 622329–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard I., Launois P., Xenarios I., Louis J. A., Acha-Orbea H., Diggelmann H.(1998). Immune response to mouse mammary tumor virus in mice lacking the alpha/beta interferon or the gamma interferon receptor. J Virol 722638–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques C. P., Kapil P., Hinton D. R., Hindinger C., Nutt S. L., Ransohoff R. M., Phares T. W., Stohlman S. A., Bergmann C. C.(2011). CXCR3-dependent plasma blast migration to the central nervous system during viral encephalomyelitis. J Virol 856136–6147. 10.1128/JVI.00202-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Tanaka N., Harada H., Kimura T., Yokochi T., Kitagawa M., Schindler C., Taniguchi T.(1999). Activation of the transcription factor ISGF3 by interferon-gamma. Biol Chem 380699–703. 10.1515/BC.1999.087 [DOI] [PubMed] [Google Scholar]
- Medina F., Segundo C., Campos-Caro A., González-García I., Brieva J. A.(2002). The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood 992154–2161. 10.1182/blood.V99.6.2154 [DOI] [PubMed] [Google Scholar]
- Metcalf T. U., Griffin D. E.(2011). Alphavirus-induced encephalomyelitis: antibody-secreting cells and viral clearance from the nervous system. J Virol 8511490–11501. 10.1128/JVI.05379-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf T. U., Baxter V. K., Nilaratanakul V., Griffin D. E.(2013). Recruitment and retention of B cells in the central nervous system in response to alphavirus encephalomyelitis. J Virol 872420–2429. 10.1128/JVI.01769-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. D., Krangel M. S.(1992). The human cytokine I-309 is a monocyte chemoattractant. Proc Natl Acad Sci U S A 892950–2954. 10.1073/pnas.89.7.2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U., Steinhoff U., Reis L. F., Hemmi S., Pavlovic J., Zinkernagel R. M., Aguet M.(1994). Functional role of type I and type II interferons in antiviral defense. Science 2641918–1921. 10.1126/science.8009221 [DOI] [PubMed] [Google Scholar]
- Nargi-Aizenman J. L., Griffin D. E.(2001). Sindbis virus-induced neuronal death is both necrotic and apoptotic and is ameliorated by N-methyl-d-aspartate receptor antagonists. J Virol 757114–7121. 10.1128/JVI.75.15.7114-7121.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargi-Aizenman J. L., Havert M. B., Zhang M., Irani D. N., Rothstein J. D., Griffin D. E.(2004). Glutamate receptor antagonists protect from virus-induced neural degeneration. Ann Neurol 55541–549. 10.1002/ana.20033 [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S., Merryman C., Benacerraf B.(1964). Electrophoretic separation and properties of mouse antihapten antibodies involved in passive cutaneous anaphylaxis and passive hemolysis. J Exp Med 120315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. J., Finley K. H.(1956). Sequelae of encephalitis; report of a study after the California epidemic. Calif Med 8498–100. [PMC free article] [PubMed] [Google Scholar]
- Palus M., Vojtíšková J., Salát J., Kopecký J., Grubhoffer L., Lipoldová M., Demant P., Růžek D.(2013). Mice with different susceptibility to tick-borne encephalitis virus infection show selective neutralizing antibody response and inflammatory reaction in the central nervous system. J Neuroinflammation 1077. 10.1186/1742-2094-10-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra B., Hinton D. R., Marten N. W., Bergmann C. C., Lin M. T., Yang C. S., Stohlman S. A.(1999). IFN-γ is required for viral clearance from central nervous system oligodendroglia. J Immunol 1621641–1647. [PubMed] [Google Scholar]
- Pearce B. D., Hobbs M. V., McGraw T. S., Buchmeier M. J.(1994). Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J Virol 685483–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewe L., Haring J., Perlman S.(2002). CD4 T-cell-mediated demyelination is increased in the absence of gamma interferon in mice infected with mouse hepatitis virus. J Virol 767329–7333. 10.1128/JVI.76.14.7329-7333.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares T. W., Marques C. P., Stohlman S. A., Hinton D. R., Bergmann C. C.(2011). Factors supporting intrathecal humoral responses following viral encephalomyelitis. J Virol 852589–2598. 10.1128/JVI.02260-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares T. W., Stohlman S. A., Hinton D. R., Bergmann C. C.(2013). Astrocyte-derived CXCL10 drives accumulation of antibody-secreting cells in the central nervous system during viral encephalomyelitis. J Virol 873382–3392. 10.1128/JVI.03307-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares T. W., DiSano K. D., Stohlman S. A., Segal B. M., Bergmann C. C.(2016). CXCL13 promotes isotype-switched B cell accumulation to the central nervous system during viral encephalomyelitis. Brain Behav Immun 54128–139. 10.1016/j.bbi.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Salamán C. R.(1996). Anorexia during acute and chronic disease. Nutrition 1269–78. 10.1016/S0899-9007(96)90702-9 [DOI] [PubMed] [Google Scholar]
- Potter M. C., Baxter V. K., Mathey R. W., Alt J., Rojas C., Griffin D. E., Slusher B. S.(2015). Neurological sequelae induced by alphavirus infection of the CNS are attenuated by treatment with the glutamine antagonist 6-diazo-5-oxo-1-norleucine. J Neurovirol 21159–173. 10.1007/s13365-015-0314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwood T. R., Morar M. M., Zellweger R. M., Miller R., May M. M., Yauch L. E., Lada S. M., Shresta S.(2012). Gamma interferon (IFN-γ) receptor restricts systemic dengue virus replication and prevents paralysis in IFN-α/β receptor-deficient mice. J Virol 8612561–12570. 10.1128/JVI.06743-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Hemming V. G., Horswood R. L., Baron P. A., Murphy B. R., Chanock R. M.(1990). Mechanism of antibody-mediated viral clearance in immunotherapy of respiratory syncytial virus infection of cotton rats. J Virol 643091–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick E. D., Leser J. S., Clarke P., Tyler K. L.(2014). Activation of intrinsic immune responses and microglial phagocytosis in an ex vivo spinal cord slice culture model of West Nile virus infection. J Virol 8813005–13014. 10.1128/JVI.01994-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan A., Ogilvie R. L., Reilly C., Abelson M. L., Raghavan S., Vasdewani J., Krathwohl M., Bohjanen P. R.(2002). Genome-wide analysis of mRNA decay in resting and activated primary human T lymphocytes. Nucleic Acids Res 305529–5538. 10.1093/nar/gkf682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna C., Bergmann C. C., Atkinson R., Stohlman S. A.(2003). Control of central nervous system viral persistence by neutralizing antibody. J Virol 774670–4678. 10.1128/JVI.77.8.4670-4678.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remakus S., Sigal L. J.(2011). Gamma interferon and perforin control the strength, but not the hierarchy, of immunodominance of an antiviral CD8+ T cell response. J Virol 8512578–12584. 10.1128/JVI.05334-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Zoecklein L. J., Howe C. L., Pavelko K. D., Gamez J. D., Nakane S., Papke L. M.(2003). Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler's murine encephalomyelitis virus infection. J Virol 7712252–12265. 10.1128/JVI.77.22.12252-12265.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg M., Kristensson K.(2002). Effects of interferon-γ on neuronal infections. Viral Immunol 15247–260. 10.1089/08828240260066206 [DOI] [PubMed] [Google Scholar]
- Rowell J. F., Griffin D. E.(1999). The inflammatory response to nonfatal Sindbis virus infection of the nervous system is more severe in SJL than in BALB/c mice and is associated with low levels of IL-4 mRNA and high levels of IL-10-producing CD4+ T cells. J Immunol 1621624–1632. [PubMed] [Google Scholar]
- Rowell J. F., Griffin D. E.(2002). Contribution of T cells to mortality in neurovirulent Sindbis virus encephalomyelitis. J Neuroimmunol 127106–114. 10.1016/S0165-5728(02)00108-X [DOI] [PubMed] [Google Scholar]
- Samuel C. E.(2001). Antiviral actions of interferons. Clin Microbiol Rev 14778–809. 10.1128/CMR.14.4.778-809.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C., Bergmann C. C.(2008). Neuroimmunology of central nervous system viral infections: the cells, molecules and mechanisms involved. Curr Opin Pharmacol 8472–479. 10.1016/j.coph.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C., Stohlman S. A., Hinton D. R., Ransohoff R. M., Cua D. J., Bergmann C. C.(2012). IFN-γ protects from lethal IL-17 mediated viral encephalomyelitis independent of neutrophils. J Neuroinflammation 9104. 10.1186/1742-2094-9-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzki B., Kieselbach B., Fisser M., Meisel C., Vogt K., Gaestel M., Lehmann M., Risch K., Grütz G., Volk H. D.(2004). IFN-γ regulation in anti-CD4 antibody-induced T cell unresponsiveness. J Am Soc Nephrol 15695–703. 10.1097/01.ASN.0000115523.50962.C0 [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E.(1987). Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236944–947. 10.1126/science.3107127 [DOI] [PubMed] [Google Scholar]
- Soh J., Donnelly R. J., Kotenko S., Mariano T. M., Cook J. R., Wang N., Emanuel S., Schwartz B., Miki T., Pestka S.(1994). Identification and sequence of an accessory factor required for activation of the human interferon gamma receptor. Cell 76793–802. 10.1016/0092-8674(94)90354-9 [DOI] [PubMed] [Google Scholar]
- Song R., Koyuncu O. O., Greco T. M., Diner B. A., Cristea I. M., Enquist L. W.(2016). Two modes of the axonal interferon response limit alphaherpesvirus neuroinvasion. MBio 7e02145-15. 10.1128/mBio.02145-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamou P., Kontoyiannis D. L.(2010). Posttranscriptional regulation of TNF mRNA: a paradigm of signal-dependent mRNA utilization and its relevance to pathology. Curr Dir Autoimmun 1161–79. 10.1159/000289197 [DOI] [PubMed] [Google Scholar]
- Steele K. E., Reed D. S., Glass P. J., Hart M. K., Ludwig G. V., Pratt W. D., Parker M. D., Smith J. F.(2007). Alphavirus encephalitides. Medical Aspects of Biological Warfare 241–270. Edited by Dembek Z. F.Fort Sam Houston, TX: Office of the Surgeon General, Department of the Army and US Army Medical Department Center & School. [Google Scholar]
- Stevens T. L., Bossie A., Sanders V. M., Fernandez-Botran R., Coffman R. L., Mosmann T. R., Vitetta E. S.(1988). Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334255–258. 10.1038/334255a0 [DOI] [PubMed] [Google Scholar]
- Stifter S. A., Bhattacharyya N., Pillay R., Flórido M., Triccas J. A., Britton W. J., Feng C. G.(2016). Functional interplay between type I and II interferons is essential to limit influenza A virus-induced tissue inflammation. PLoS Pathog 12e1005378. 10.1371/journal.ppat.1005378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C. L., Wilson T. J., Strauch P., Colonna M., Pelanda R., Torres R. M.(2010). Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med 2071485–1500. 10.1084/jem.20092695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szomolanyi-Tsuda E., Welsh R. M.(1996). T cell-independent antibody-mediated clearance of polyoma virus in T cell-deficient mice. J Exp Med 183403–411. 10.1084/jem.183.2.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau G., Rothman P.(1999). Biologic functions of the IFN-γ receptors. Allergy 541233–1251. 10.1034/j.1398-9995.1999.00099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J. R., Verhoeven D., Page C. A., Turner D., Farber D. L.(2010). Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol 849217–9226. 10.1128/JVI.01069-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangarajh M., Masterman T., Hillert J., Moerk S., Jonsson R.(2007). A proliferation-inducing ligand (APRIL) is expressed by astrocytes and is increased in multiple sclerosis. Scand J Immunol 6592–98. 10.1111/j.1365-3083.2006.01867.x [DOI] [PubMed] [Google Scholar]
- Tran E. H., Prince E. N., Owens T.(2000). IFN-γ shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol 1642759–2768. 10.4049/jimmunol.164.5.2759 [DOI] [PubMed] [Google Scholar]
- Trujillo J. A., Fleming E. L., Perlman S.(2013). Transgenic CCL2 expression in the central nervous system results in a dysregulated immune response and enhanced lethality after coronavirus infection. J Virol 872376–2389. 10.1128/JVI.03089-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschen S. I., Stohlman S. A., Ramakrishna C., Hinton D. R., Atkinson R. D., Bergmann C. C.(2006). CNS viral infection diverts homing of antibody-secreting cells from lymphoid organs to the CNS. Eur J Immunol 36603–612. 10.1002/eji.200535123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor W. R., Wesselingh S., Levine B., Griffin D. E.(1992). Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J Immunol 1494016–4020. [PubMed] [Google Scholar]
- van den Hurk A. F., Ritchie S. A., Mackenzie J. S.(2009). Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol 5417–35. 10.1146/annurev.ento.54.110807.090510 [DOI] [PubMed] [Google Scholar]
- Vences-Catalán F., Santos-Argumedo L.(2011). CD38 through the life of a murine B lymphocyte. IUBMB Life 63840–846. 10.1002/iub.549 [DOI] [PubMed] [Google Scholar]
- Villari P., Spielman A., Komar N., McDowell M., Timperi R. J.(1995). The economic burden imposed by a residual case of eastern encephalitis. Am J Trop Med Hyg 528–13. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Reisen W. K.(2010). Present and future arboviral threats. Antiviral Res 85328–345. 10.1016/j.antiviral.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg D. O., Fordham S., Bernard C. C., Cowden W. B., Ramshaw I. A.(1996). IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol 1573223–3227. [PubMed] [Google Scholar]
- Young H. A., Bream J. H.(2007). IFN-γ: recent advances in understanding regulation of expression, biological functions, and clinical applications. Curr Top Microbiol Immunol 31697–117. [DOI] [PubMed] [Google Scholar]