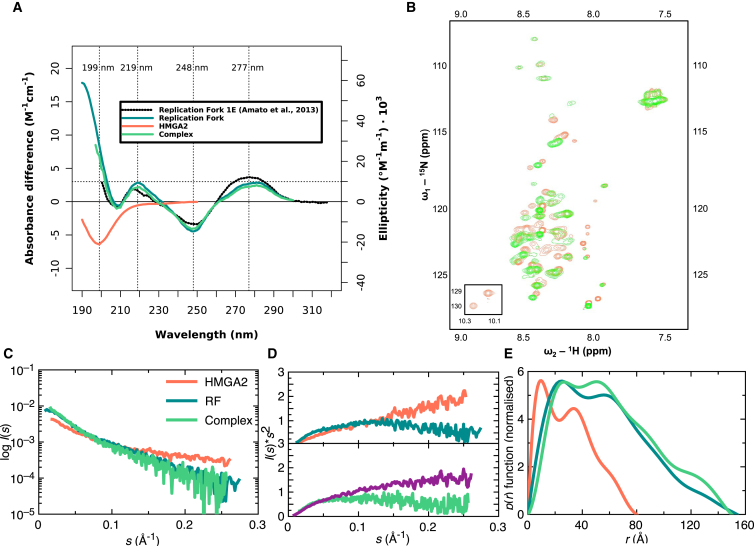
Figure 5.
(A) Given here is the far-UV CD spectra of HMGA2, RF, and complex displayed as an average of three measurements indicating disordered HMGA2 and confirmation of RF formation. A reference RF (56) (dotted black) spectrum was overlaid onto the experimental RF (teal) for comparison. (B) Shown here is the 15N HSQC spectral overlay of free HMGA2 (coral) with HMGA2 in complex with RF (green), suggesting a conformational change of HMGA2 upon interaction with RF. (C) Given here is merged SAXS data of HMGA2 (coral), RF (teal), and complex (sea green) obtained from multiple concentrations. The corresponding (D) Kratky plot suggests that HMGA2 (coral) is a disordered protein and that RF (teal) is structured (upper panel). Theoretical Kratky analysis of the complex (purple) is distinctly different from the experimental analysis (sea green), suggesting that upon interaction with RF, HMGA2 undergoes a conformational change (lower panel). (E) Given here is the P(r) function of HMGA2, RF, and complex (same color scheme as C and D) outlining the multidomain nature of the molecules and their Dmax. To see this figure in color, go online.