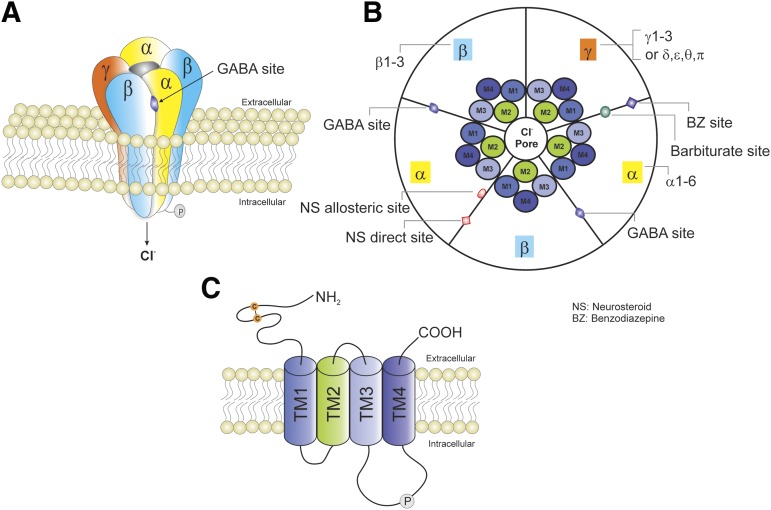
Fig. 1.
Schematic representation of typical GABA-A receptor (GABA-AR) structure and subunit composition. (A) GABA-ARs are heteropentamers forming a channel that is permeable to chloride ion passage. (B) A top view of the pentamer. GABA-ARs are made from a repertoire of 19 known subunits: α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3. The most general stoichiometry of GABA-ARs contains two αs, two βs, and one γ; and the γ subunit can be substituted by δ, ε, θ, or π. Each subunit has four TMs (TM1–TM4). TM2s form a selective channel pore. GABA exerts fast inhibitory actions by activating postsynaptic GABA-ARs in the brain, causing the influx of negatively charged chloride ions and hyperpolarization of neurons, which serve to reduce neuronal excitability and firing. The GABA binding sites are located at the junction between subunit α and β, whereas benzodiazepines (BZs) bind at the interface between subunits α and γ. Barbiturates binding sites are distinct from the BZ binding site. The NSs have two putative binding sites including allosteric and direct binding sites. The allosteric binding site is located at the α subunit TMs, whereas the direct binding site is within the TMs of the α and β subunits. (C) GABA-ARs belong to the Cys-loop family of ligand-gated ion channels, which also contains nicotinic acetylcholine, glycine, and serotonin 5-HT3 receptors. Each subunit has one long extracellular N terminus that interacts with a variety of drugs including BZs, barbiturates, and NSs; four TMs (TM1–TM4); and one short intracellular loop that links TM1 and TM2, one short extracellular loop that links TM2 and TM3, one long intracellular loop that links TM3 and TM4 and can be modulated by phosphorylation, and one small extracellular C terminus.