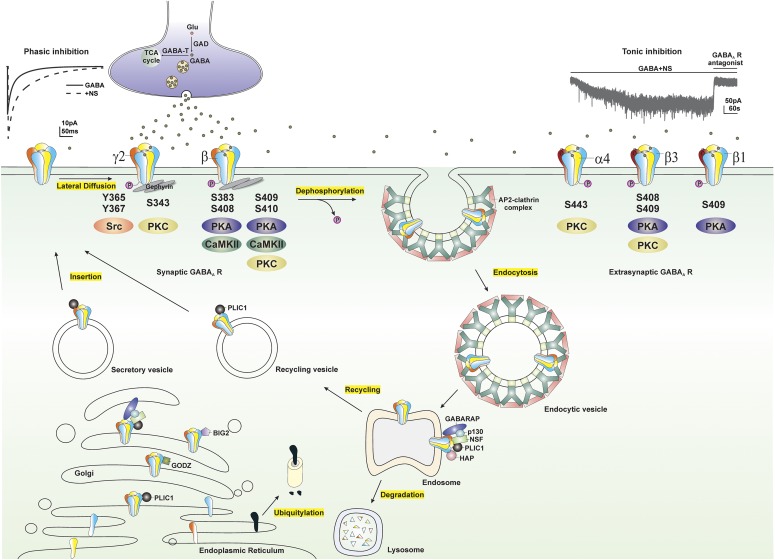
Fig. 5.
Modulation of extrasynaptic GABA-A receptors (GABA-ARs) trafficking by phosphorylation. GABA-ARs composed of α, β, and γ subunits are mostly clustered at synaptic sites, whereas GABA-ARs composed of α, β, and δ subunits are clustered at extrasynaptic sites. GABA-ARs are assembled in the ER, matured in the Golgi, secreted, and inserted into the plasma membrane. Synaptic GABA-ARs, reaching their destination through lateral diffusion in the cell membrane, are activated by presynaptic release of GABA and produce fast and transient phasic inhibition, whereas continuous activation of extrasynaptic GABA-ARs by ambient GABA generates persistent tonic inhibition that sets the baseline of neuronal inhibition. NSs at submicromolar concentrations potentiate both phasic and tonic inhibition through allosteric modulation of synaptic and extrasynaptic GABA-ARs, respectively. GABA-AR subunits, including α4, β, and γ2 subunits, contain residues that can be phosphorylated by various protein kinases. Phosphorylation of residues within GABA-ARs not only regulates receptor function but maintains their surface expression. Dephosphorylation of these subunits triggers receptor internalization through adaptor protein 2 (AP2)-clathrin complex–dependent endocytosis. Internalized receptors are subsequently transported to the endosomal system, where they can be either recycled to the surface or degraded in the lysosomes. GABA-AR trafficking is facilitated by a variety of protein-protein interactions. BIG2, a brefeldin A-inhibited GDP/GTP exchange factor, binds to the intracellular loop of β subunits and is involved in the trafficking of receptors; GABARAP, a GABA-AR–associated protein, interacts with the γ2 subunit of GABA-ARs and might facilitate receptor insertion into the cell membrane; GABA-T, GABA transaminase; GODZ, a Golgi-specific DHHC zinc finger domain protein, mediates post-translational palmitoylation; HAP, a Huntingtin-associated protein, interacts with the β subunit of GABA-ARs and facilitates receptor recycling to the cell membrane; GAD, glutamate decarboxylase; NSF, a N-ethylmaleimide–sensitive factor, interacts with GABARAP and p130 and plays a crucial role in intracellular transport; p130, a phospholipase, shares the same binding site on the γ2 subunit with the GABARAP; PLIC-1, a ubiquitin-like protein, increases receptor stability and enhances membrane insertion of receptors.