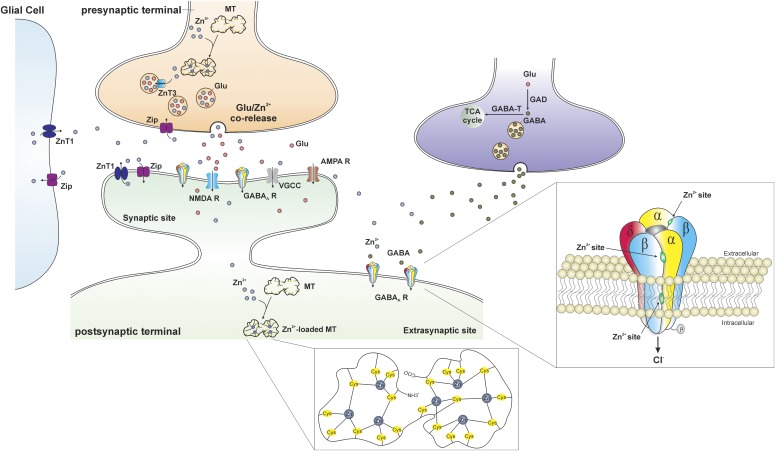
Fig. 6.
Trafficking of Zn2+ at the gluzinergic synapse. In the gluzinergic terminal, the availability of free Zn2+ is regulated by metallothioneins (MTs), the primary intracellular Zn2+-buffering proteins. The MT is a dumbbell-shaped, cysteine (Cys)-rich protein composed of two domains in which 7 zinc atoms are tetrahedrally bound to 20 cysteines (inset, middle). The Zn2+ transporter (ZnT) and Zip proteins are also involved in the regulation of Zn2+ in the cytoplasm. ZnT proteins promote Zn2+ efflux and vesicular uptake to decrease the amount of intracellular Zn2+, whereas Zip proteins facilitate the influx of extracellular Zn2+ into neurons and glial cells to increase the concentration of intracellular Zn2+. Free Zn2+ is transferred into synaptic vesicles through the ZnT3 proteins and stored with glutamate. During normal neurotransmission, Zn2+- and glutamate-containing vesicles fuse with cell membrane and corelease Zn2+ and glutamate into the synaptic cleft. There are a variety of postsynaptic targets that Zn2+ can act on, including N-methyl-d-aspartate receptors (NMDA Rs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA Rs), voltage-gated calcium channels (VGCCs), GABA-ARs, and a number of other channels, transporters, and receptors. Three distinct Zn2+ binding sites mediate its inhibition of extrasynaptic δ-containing GABA-ARs (inset, right): one site at the internal surface of the channel pore and two at the external amino terminus of the α-β interfaces.