Extended Data Figure 3. Phosphorylation of Tyr-122 of PIN4 by F3-T3.
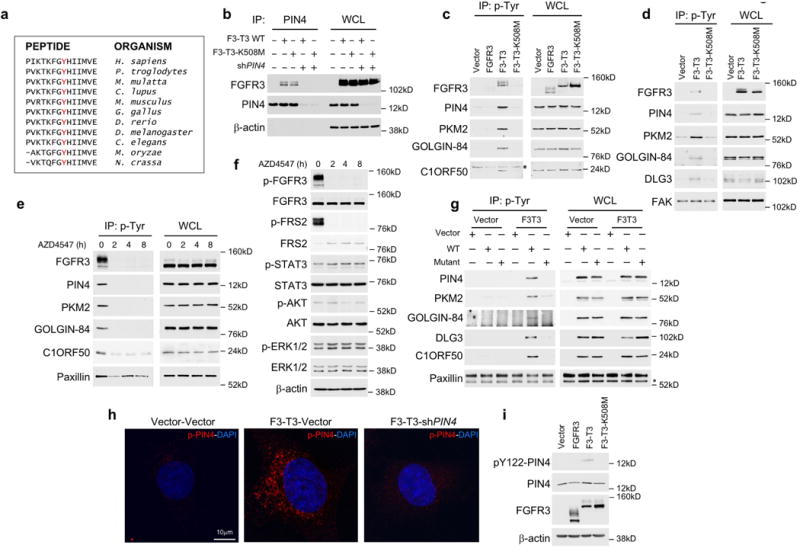
a, Amino acid sequence flanking Tyr-122 of PIN4 (in red) is evolutionarily conserved. b, Immunoprecipitation-western blot analysis of HA-F3-T3 or F3-T3-K508M with or without silencing of endogenous PIN4. β-actin is shown as loading control. WCL, whole cell lysates. c, Immunoblot analysis of phosphotyrosine immunoprecipitates from U87 glioma cells expressing empty vector, FGFR3, F3-T3 or F3-T3-K508M using the indicated antibodies (left panels). WCL, right panels. The asterisk indicates a non-specific band. d, Immunoblot analysis of phosphotyrosine immunoprecipitates from HA expressing empty vector, F3-T3 or F3-T3-K508M using the indicated antibodies (left panels); WCL, right panels. FAK is shown as loading control. e, Immunoblot analysis of phosphotyrosine immunoprecipitates from GSC1123 harboring endogenous F3-T3 shows a decreased phosphorylation of F3-T3 substrates following treatment with AZD4547 for the indicated times (left panels); WCL, right panels. Paxillin is shown as loading control. f, Immunoblot analysis of canonical FGFR signaling proteins in GSC1123 treated with AZD4547 for the indicated time. β-actin is shown as loading control. g, Immunoblot analysis of phosphotyrosine immunoprecipitates from HA-F3-T3 or HA-vector transduced with WT or the unphosphorylable (Y/A) F3-T3 kinase substrate mutants. Paxillin is shown as loading control. The asterisk indicates a non-specific band. h, Confocal images of immunofluorescence staining using the pY122-PIN4 specific antibody (red) in HA transduced with vector or F3-T3 without or with silencing of endogenous PIN4. Nuclei were stained with DAPI (blue). i, Immunoblot analysis of pY122-PIN4, total PIN4 and FGFR3 in SF126 cells transduced with FGFR3, F3-T3, F3-T3-K508M or the empty vector. β-actin is shown as loading control. Molecular weights are indicated in all panels. Experiments in b-g, and i were repeated independently three times with similar results. Experiment in h was repeated independently four times with similar results.
