Figure 6.
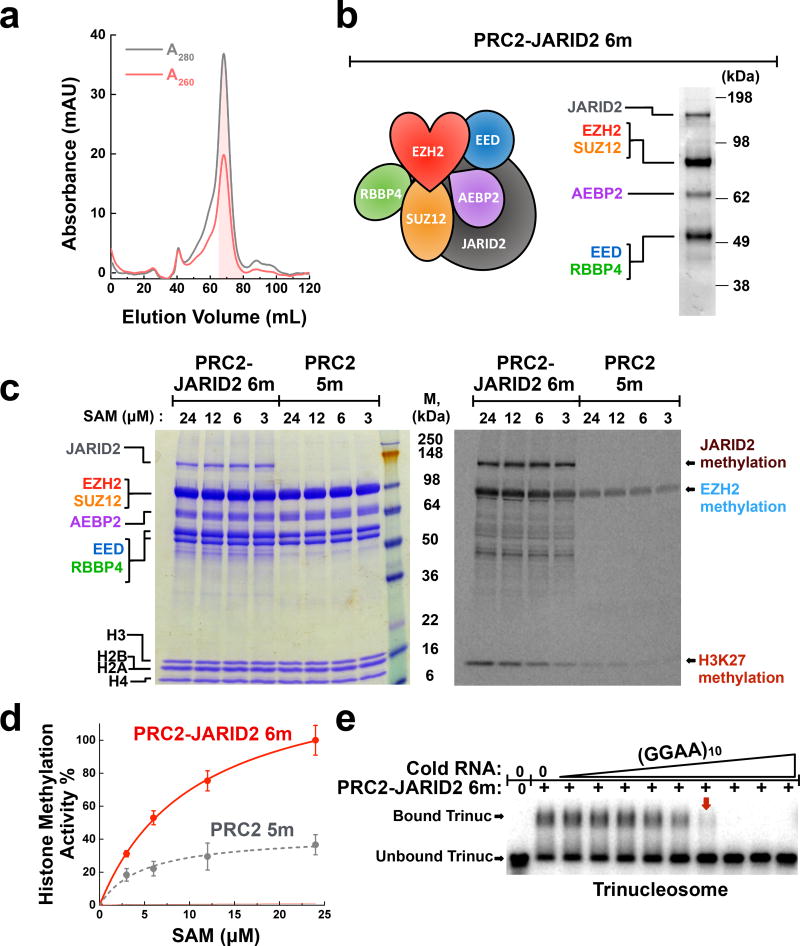
JARID2 enhances PRC2 histone methyltransferase activity but does not prevent eviction by RNA. (a) Fractionation of PRC2−JARID2 6-mer over Sephacryl S-400 sizing column shows that the complex is monodisperse. A260/A280 ratio <0.7 indicates the lack of nucleic acid contamination. Shaded fractions were collected. (b) Typical SDS-PAGE gel showing the purity of recombinant PRC2-JARID2 6-mer complex. (c) A comparison of histone methyltransferase activity between PRC2−JARID2 6-mer and PRC2 5-mer using mononucleosome substrates. PRC2 and histone proteins visualized by Coomassie-staining (left-hand gel); methylation signal detected by 14C-autoradiography (right-hand gel). (d) Quantification of H3 methyltransferase activity. Error bars give SD, n = 3 independent experiments. (e) (GGAA)10 RNA disrupts pre-formed complexes of PRC2−JARID2-trinucleosome. Complexes were fully disrupted (red box arrow) at a PRC2:RNA molar ratio of ~2:1. Samples represent 3-fold titrations ranging from 0–10 µM RNA. Uncropped gel images are shown in Supplementary Data Set 1.