Figure 4. Mechanosensory responses depend largely on synaptic input via gap junctions.
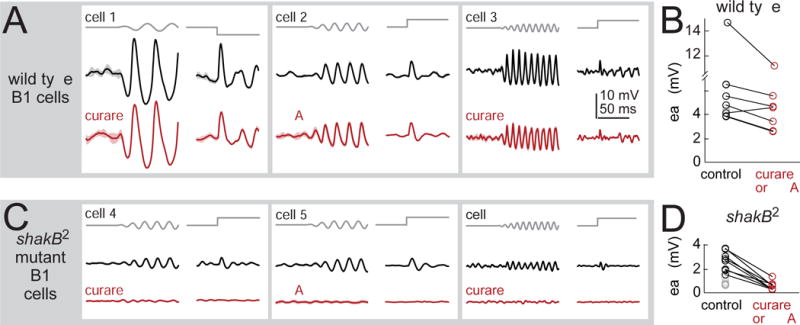
(A) Stimulus-evoked responses of three example B1 cells recorded in wild type flies, before and after blocking nicotinic receptors (with 50 μM curare or 0.5 μM MLA). Vibration frequency was chosen to match the cell’s preferred frequency (25, 50, and 100 Hz) and the direction of the step stimulus was chosen to match the cell’s preferred direction (1.5 μm vibration, 3 μm step). Shaded bands are SEM across trials. Cells were recorded in the GMR45D07-Gal4 line, which labels a mixture of B1 cell types (B1-high, -mid, and -low).
(B) Peak amplitude of B1 responses to the step stimulus. Blocking nicotinic receptors produces a small but significant effect (20 ± 6% reduction, p<0.05, paired two-sided signed-rank test, n=7 cells).
(C) B1 cells in the shakB2 gap junction subunit mutant. Blocking nicotinic receptors essentially abolished stimulus-evoked responses. Sinusoids were 50 or 100 Hz, and all cells were recorded in GMR45D07-Gal4.
(D) Peak amplitude of B1 responses to the step stimulus in shakB mutants. Responses were significantly smaller than wild type (p<10−3, two-sided ranksum test; n=10 wild type cells and n=15 mutant cells). In shakB mutants, nicotinic antagonists reduced responses by 78±4% (p<10−3, paired two-sided signed-rank test; n=8 mutant cells). We corrected p-values for multiple comparisons using a Bonferroni-Holm procedure (3 tests in this figure). Gray symbols are experiments where antagonists were not tested.
