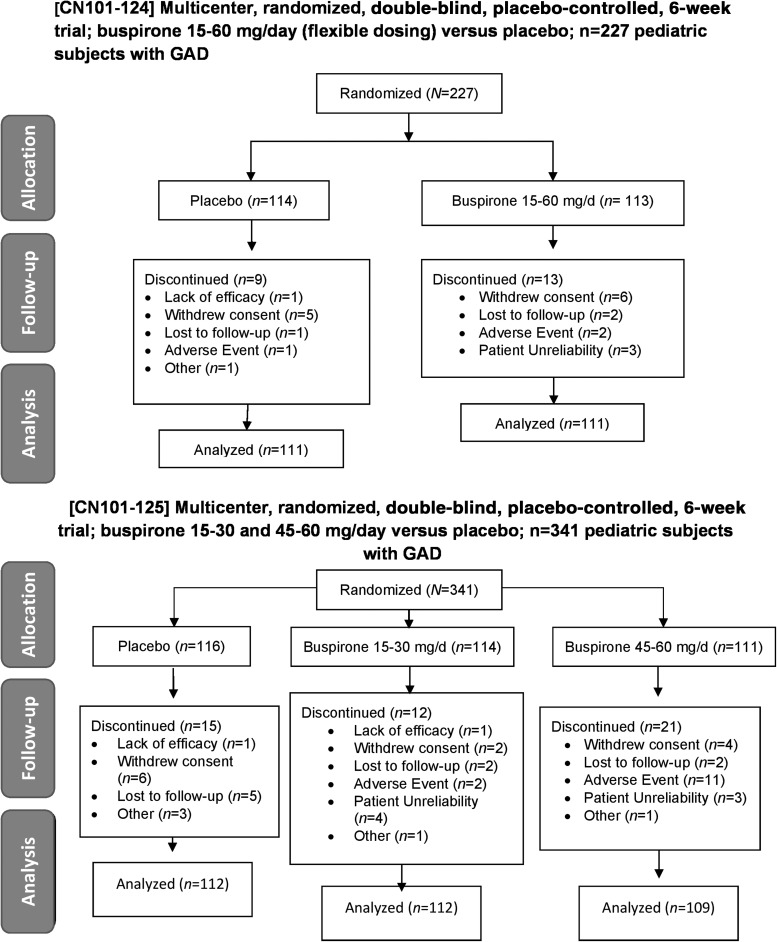
FIG. 1.
CONSORT diagrams for efficacy studies in children and adolescents with GAD. Study CN101-124 (top panel) was a 6-week, multicenter, randomized, double-blind, placebo-controlled trial of buspirone 15–60 mg per day versus placebo in children and adolescents with GAD. Study CN101-125 was a 6-week, multicenter, randomized, double-blind, placebo-controlled trial of buspirone 15–30 mg per day and 45–60 mg per day versus placebo in children and adolescents with GAD (bottom panel). GAD, generalized anxiety disorder.