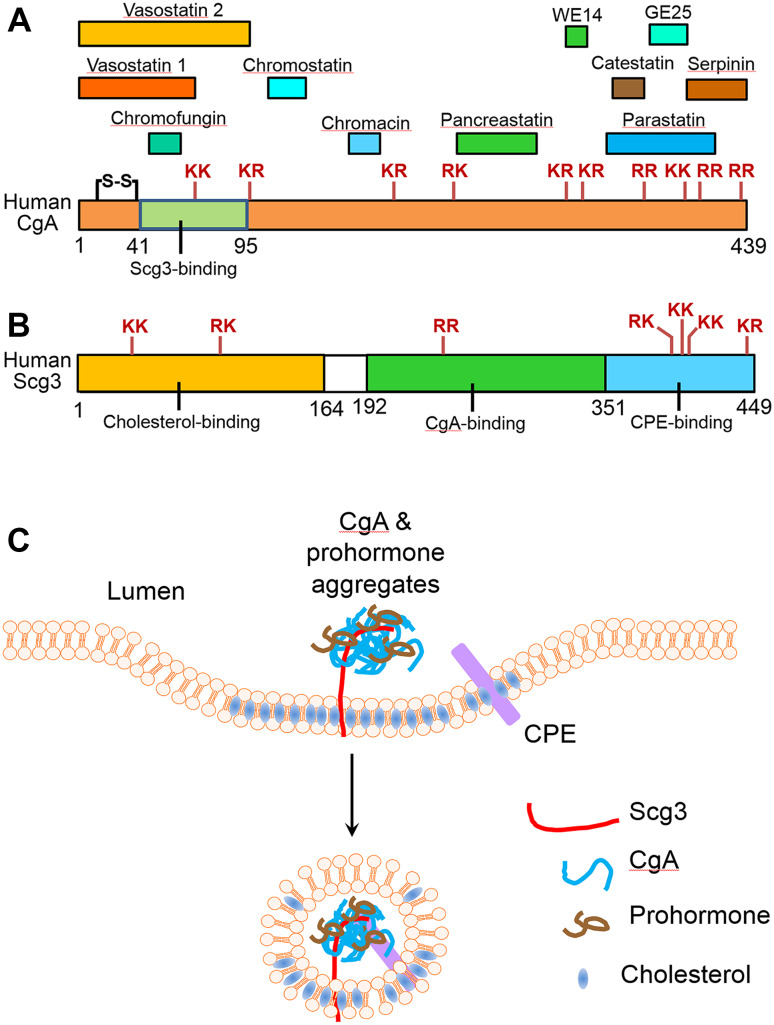
Fig. 1.
Human CgA (hCgA) and Scg3, their functional domains, related bioactive peptides, and role in the biogenesis of secretory granules. a Mature hCgA without the signal peptide is 439 amino acids (aa) with a disulfide bond. The positions of ten dibasic peptides are indicated. hCgA is the precursor for at least 11 biologically active peptides: vasostatin I (CgA1–76), vasostatin II (CgA1–131), pancreastatin (CgA250–301), catestatin (CgA352–372), WE14 (CgA324–337), chromofungin (CgA47–66), parastatin (CgA347–419), chromacin (CgA176–197), serpinin (CgA411–436), chromostatin (CgA124–143), and GE25 (CgA375–399). b Mature hScg3 without the signal peptide is 449 aa. No cysteine residue. The positions of seven dibasic peptides are indicated. There is currently no known hScg3-derived biologically active peptide. Scg3 has three functional domains: cholesterol-binding domain (1–164), CgA-binding domain (192–351), and CPE-binding domain (352–449). c A model for Scg3 to regulate the biogenesis of secretory granules. CgA forms aggregates with prohormones in the lumen of the TGN. Scg3 as a linker simultaneously binds to CgA in the aggregates and the cholesterol-rich membrane domains of the TGN. Scg3 also interacts with CPE in the membrane. The complex forms a dense core secretory granule (DCG), which is engulfed by the TGN membrane and released as secretory vesicles