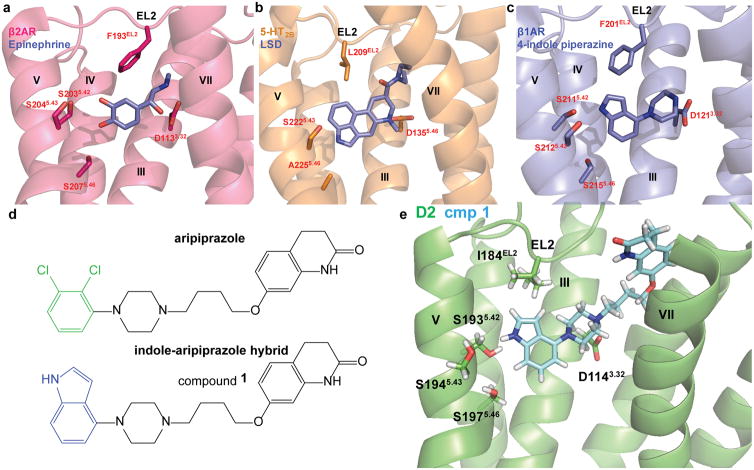
Figure 1. Structure-Inspired Design of Indole-Aripiprazole Hybrid Ligands.
D2 ligand design based on comparison of three aminergic crystal structures. a) β2 adrenergic receptor nanobody-stabilized with epinephrine bound (4LDO) indicates the catechol of epinephrine is involved in an extensive hydrogen bond network with transmembrane (TM) 5 serines. b) Structure of the 5-HT2B receptor with LSD bound (5TVN) indicates that EL2.52 Leu209 forms hydrophobic cap over ligand, preventing ligand egress. c) Thermostabolized β1 adrenergic receptor with 4-indole piperazine bound (3ZPQ) shows that the indole N-H interacts with Ser5.42 in a hydrogen bond d) Design of indole-aripiprazole hybrid compounds by addition of 4-indole (blue) replacing the dichlorophenyl (green) of aripiprazole resulting in compound 1. e) Docking of 1 in D2 homology model places the unsubstituted 4-indole moiety of the indole-aripiprazole hybrid 1 in the D2 orthosteric binding pocket making contact with TM5 Ser5.42.