Abstract
Background
Anesthetics are believed to alter functional connectivity across brain regions. However, network level analyses of anesthesia, particularly in humans are sparse. We hypothesized that propofol-induced loss of consciousness results in functional disconnection of human sensorimotor cortices underlying the loss of volitional motor responses.
Methods
We recorded local field potentials from sensorimotor cortices in patients with Parkinson disease (N =12) and essential tremor (N =7) undergoing deep brain stimulation surgery, before and after propofol-induced loss of consciousness. Local spectral power and inter-regional connectivity (coherence and imaginary coherence) were evaluated across conditions for the two populations separately.
Results
Propofol anesthesia caused power increase for frequencies between 2–100 Hz across the sensorimotor cortices and a shift of the dominant spectral peak in α and β frequencies toward lower frequencies (Median ±SD peak frequency: 24.5 ±2.6 Hz to 12.8 ±2.3 Hz in Parkinson disease and 13.8 ±2.1 Hz to 12.1 ±1.0 Hz in essential tremor). Despite local increases in power, sensorimotor cortical coherence was suppressed with propofol in both cohorts, specifically in β frequencies (18–29 Hz) for Parkinson disease and α and β (10–48 Hz) in essential tremor.
Conclusion
The decrease in functional connectivity between sensory and motor cortices despite an increase in local spectral power suggests propofol causes a functional disconnection of cortices with increases in autonomous activity within cortical regions. This pattern occurs across diseases evaluated, suggesting these may be generalizable effects of propofol in patients with movement disorders and beyond. Sensoriomotor network disruption may underlie anesthetic-induced loss of volitional control.
Keywords: Anesthesia, Propofol, Sensorimotor Cortex, Parkinson disease, Essential tremor
Introduction
Propofol acts through GABAergic inhibition of neurons within the cortex, thalamus, brainstem, and spinal cord1,2 leading to unconsciousness by shifting cortical dynamics such that neuronal networks become functionally isolated in time and space3,4. Because neuronal networks are believed to communicate via synchronized oscillations, this functional isolation is most likely manifested as loss of connectivity across brain regions5,6.
Electroencephalography (EEG) studies indicate enhancement of α (8–12 Hz) and attenuation of β (13–30 Hz) oscillations as major features of propofol-induced unconsciousness3,7–10. Propofol also shifts the spatial distribution of α oscillations from an occipital focus in an awake state to a frontal focus in an unconscious state11. These frontal dominant α oscillations may emerge from the potentiation of GABAA synaptic currents that alter thalamocortical connectivity12, including concurrent disruption of normal α rhythms in the posterior-projecting thalamic nuclei and the emergence of α activity in frontothalamic nuclei13. Dynamic causal modeling also suggests changes in cortical connectivity in propofol-induced unconsciousness, with a reduction in frontal-to-parietal connectivity but preserved parietal-to-frontal connectivity14,15.
The current study of propofol-induced changes in sensorimotor cortices was motivated by the propofol-related loss of motor responses16. General anesthetics such as propofol seem to disrupt cortical integration of incoming information17–19. While external sensory stimuli can activate the cortex in an anesthetized state, these stimuli fail to be experienced, suggesting a differential effect of anesthetics on functionally discrete cortices14,20,21. A non-human primate study of propofol revealed disruption of sensorimotor/premotor coherent β oscillations preceding loss of consciousness suggesting that the transition to propofol-induced unconsciousness is associated with disruption of regional interactions between sensory and higher order cortical networks17. This is consistent with the role of α and β oscillations in mediating inter-cortical connectivity22 and that these oscillations, to some extent, impart an inhibitory control on brain circuits23. In the motor network, sensorimotor cortical synchrony has been reported in these frequencies, both within and across cortices and in relation to movement24,25. This synchrony is important for sensorimotor integration and formation of motor memories26, which are higher level functions lost under anesthesia. Likewise, both α and β oscillations are associated with the “akinetic” state in motor networks27–29, inhibiting movement by modulating pyramidal tract neuronal firing rate24. It follows that propofol may result in modulation of these oscillatory signals both intra- and inter-regionally.
Although EEG studies have been critical in this field, electrocorticography (ECoG) provide increased spectral sensitivity and spatial resolution. Importantly, the spatial specificity of ECoG enables us to distinguish M1 from S1, which is not possible with EEG. Moreover, M1 and S1 are reciprocally connected and hence make an interesting model to study corticocortical communication. We employed ECoG during deep brain stimulation (DBS) implantation surgery in subjects with either Parkinson disease or essential tremor in order to delineate common patterns of change associated with propofol administration despite disease-specific cortical pathophysiology. We hypothesized that similar to the findings of propofol-induced diminished connectivity between subcortical and cortical brain activity30, propofol administration decreases sensorimotor cortical α and β connectivity in cortices, signifying a functional disconnection of them.
Materials and methods
Patients and surgery
Twelve subjects with idiopathic Parkinson disease and seven subjects with essential tremor undergoing bilateral implantation of DBS leads between January 2014 and October 2016, were included in this study. No a priori power calculation was conducted to guide the sample size and recruitment was performed in accordance with our previous experience with intraoperative electrophysiological studies from patients with movement disorders31,32. All subjects provided written informed consent approved by the institutional review board at the University of California, Los Angeles.
All long-acting and short-acting Parkinson disease-related medications were withdrawn at least 24 hours and 12 hours prior to the surgical procedure, respectively. In patients with Parkinson disease, the DBS leads were targeted to motor region of Globus Pallidus internus (GPi, 2–4 mm anterior, 19–24 mm lateral and 4–6 mm inferior to the mid-commissural point) with intraoperative microelectrode recording (MER) confirmation. In patients with essential tremor, the ventral intermediate nucleus (ViM) of the thalamus was targeted for DBS electrode implantation (6 mm anterior to posterior commissure (PC), 11 mm lateral to the 3rd ventricular wall, and at the anterior commissure (AC)-PC line). All trajectories were confirmed with intraoperative awake macro-stimulation testing.
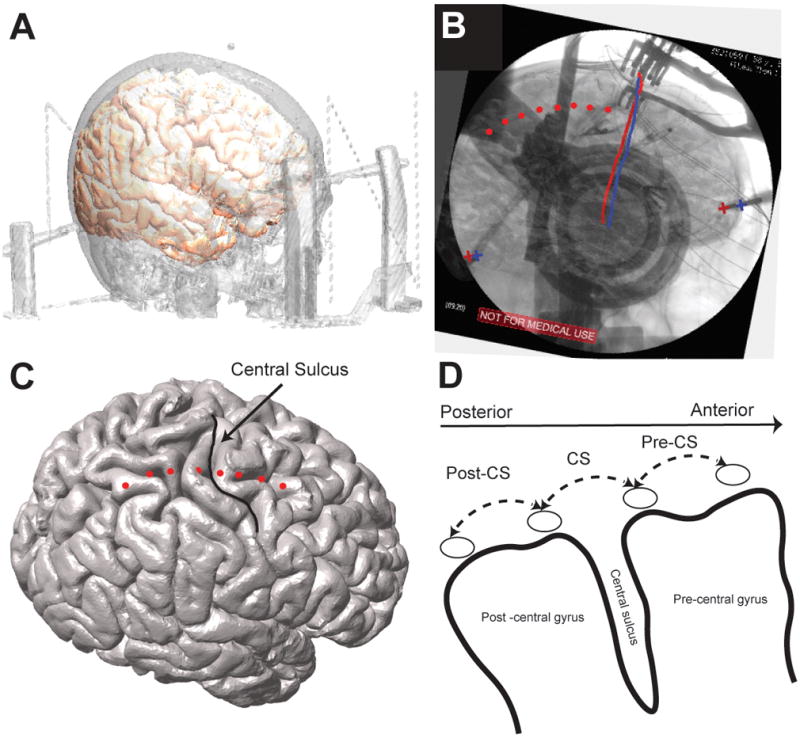
Prior to right-sided DBS lead implantation, an 8-contact ECoG strip (platinum-iridium 4 mm contacts with 1 cm spacing, AdTech Medical) was implanted subdurally via the right frontal burr hole placed for DBS implantation. The burr hole was always at or up to 1 cm in front of the coronal suture. Given central sulcus is located in 3–5 cm behind the coronal suture, an eight-contact ECoG strip ensured coverage of the central sulcus through the burr hole. After implantation of the DBS electrode in each hemisphere, a single view lateral fluoroscopy image was acquired to confirm DBS electrode placement. After ECoG recordings were acquired, the subdural strip was removed, the DBS electrode was locked into place and the burr hole was sealed. Following the procedure, a post-operative CT scan was obtained for confirmation of DBS electrode position. A detailed description of localization of ECoG strip and signals utilized in this study is provided as supplemental digital content. We used three bipolar contact pairs for analyses: CS (spanning the central sulcus), Post-CS (immediately posterior to CS), and Pre-CS (immediately anterior to CS) (Figure 1).
Figure 1.
Localization of cortical ECoG strip and cortical signals used for analysis (A) registration of pre-operative structural high resolution T1 weighted MRI and CT to co-localize cortical brain surface and skull (B) Tips of the stereotactic frame and DBS leads (marked by + signs and straight red and blue lines on the image, respectively) are used as landmarks to complete 2D-3D fusion of fluoroscopic image and cortical surface. Once the fusion is complete cortical contacts (visible on the fluoroscopic image) are marked manually on the fused images. (C) Marked ECoG contacts are shown on cortical surface and identified relative to the central sulcus. (D) Three cortical bipolar signals spanning post to pre central gyri/sensorimotor cortices (posterior to anterior: PostCS, CS and PreCS) are marked and used for all of the analyses. (For more detailed description of the registration method please refer to the online supplementary digital content)
Data recording and pre-processing
ECoG recordings were obtained with a scalp ground and reference. Signal acquisition was performed using BCI2000 v2 connected to a g.USBamp 2.0 amplifier at a sampling rate of 2400 Hz (g. tec Austria). Prior to recordings, patients were off propofol (and all anesthetics) for at least 1.5 hours. In each subject, bilateral DBS LFPs (GPi for Parkinson disease and ViM for essential tremor) and simultaneous frontoparietal ECoG signals were initially recorded while subjects performed six blocks of alternating rest and finger tapping. We began recordings with patients resting awake with eyes open for one minute after which propofol was administered according to the attending anesthesiologist’s clinical judgment. Recordings continued and patients were assessed verbally at least every 30 seconds after propofol administration to ensure and determine the timing of loss of responsiveness. We used the modified observer’s assessment of alertness/sedation scale (MOAA/S) to evaluate the subject’s level of alertness33. Each patient included in the study reached score 0/1, after which recordings continued for one minute. Recordings continued on average for 5 minutes after the start of propofol administration. Due to variations in propofol dosing, cardiac output, and blood volume across subjects, we expected substantial differences in circulation time and anesthetic induction across subjects. Therefore we focused our analyses on pre-bolus and post-induction steady state signals, using the last minute of recording as the time period when subjects were completely anesthetized and no longer verbally responsive (herein referred to Anesthesia period (Anes)) and contrasted this with the Pre-Anesthesia (PreAnes) stage.
Signal analysis was performed in MATLAB (Version 8.6, The Mathworks Inc., Natick, MA), using Fieldtrip toolbox for EEG/MEG-analysis34 and the Chronux toolbox35. Bipolar re-referencing of adjacent contacts was used to emphasize local and minimize global signals. Raw ECoG data were first subjected to automatic artifact rejections and subsequently verified through visual inspection with an interactive waveform browser. Automatic artifact detection was accomplished by first performing a full-wave rectification, then taking the first derivative, and subsequently filtering with a five-point median filter to identify waveform segments that exceeded five standard deviations of the entire recorded session for each subject. Location windows of artifact segments were extended by 2 ms on each side. Artifact segments were then replaced using a piecewise cubic interpolation based on surrounding data. Pre-processed data were band pass filtered for frequencies between 1–500 Hz using two-way least-squares FIR filtering (eegfilt.m). Line noise and its harmonics (up to 500 Hz) were removed from the data using notch filter implemented in fieldtrip toolbox. Of note, while deep brain LFP were also recorded, the focus of the current study was on cortical dynamics. Therefore, analysis of these signals is deferred.
Power Spectral Density (PSD) and spectral peak
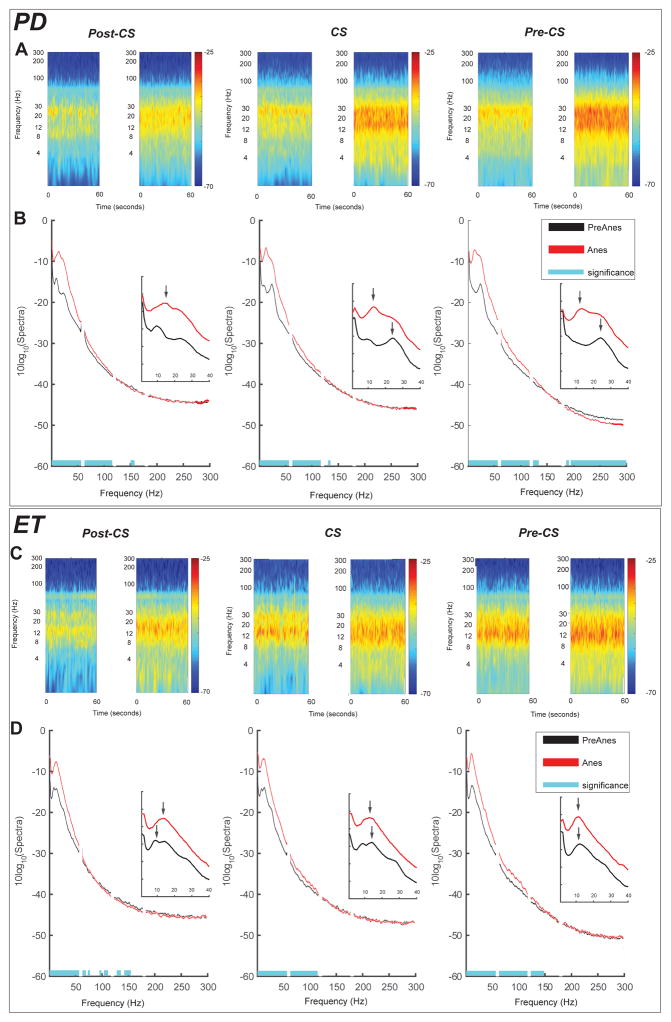
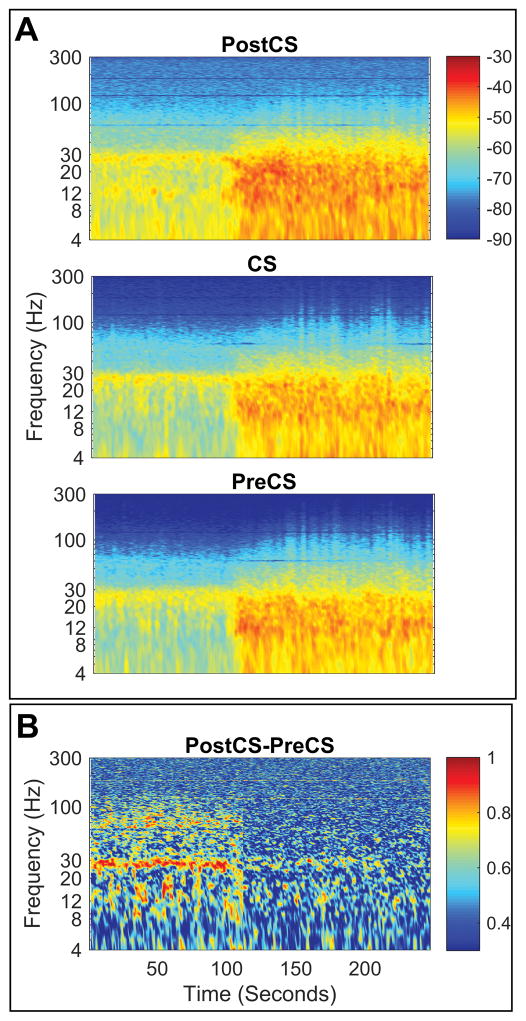
Time frequency representation of power (TFR) for PreAnes and Anes conditions (1 min each) was calculated using the multitaper method36 in 1 second consecutive time windows with no overlap for frequencies of 1 to 300 Hz with ±2 Hz frequency bandwidth (3 tapers). Figure 2A shows TFR power maps for an example subject from Parkinson disease cohort. Average TFR maps for both conditions were then calculated for each cohort at Post-CS, CS and Pre-CS. Tapered spectra likewise were derived using similar parameters as TFR maps. To account for inter-subject variability in baseline power, each segmented spectra was normalized to the total power of the signal during PreAnes (excluding the line noise and its multiples). To evaluate changes in the dominant spectral peak frequencies with propofol, we averaged such dominant peaks for all three cortical signals to derive a single average peak frequency value per condition per subject. Such average peak frequencies were then compared between the two groups.
Figure 2.
Changes in sensorimotor power and coherence in an example subject (A) Time frequency representation of power for three cortical signals investigated (top to bottom) indicating local power increase for frequencies <100 Hz similarly at all sensorimotor cortices. Dashed vertical line indicates timing of propofol injection (bolus) (B) Time frequency representation of coherence (i.e. coherogram) between signals recorded from Pre and Post central gyri (PreCS and PostCS, respectively) from the sample subject as in panel (A) indicating de-coherence in beta (13–30 Hz) between sensory and motor cortices.
While our primary focus is on contrasting Anes and PreAnes conditions, we also preliminary investigated relative temporal (as opposed to the absolute timing of) changes in spectral power across frequency bands. To do this, we analyzed TFR maps for the entire recording for each subject, defining average power over time for the frequency bands: δ (1–4 Hz), θ (4–8 Hz), α (8–12 Hz), β (13–30 HZ), γ (40–100 Hz). More controlled studies with weight and cardiac-output adjusted dosing will be required in the future for more dynamic assessment of propofol effects.
Coherence and Imaginary Coherence
To describe the degree of co-variability between the two signals, we estimated magnitude squared coherence between Pre-CS and Post-CS (covering motor/premotor and sensory cortices respectively and do not share a common contact) with 1 Hz frequency resolution. Magnitude squared coherence was calculated using multitaper method with time window and frequency smoothing parameters identical to the analysis of power spectral density (frequencies of 1 to 300 Hz with ±2 Hz frequency bandwidth (3 tapers)). Figure 2B shows sample coherogram (time frequency representation of coherence) for one subject (same as Figure 2A) for the entire period of recording.
We further explored effects of spurious volume conduction on coherence using imaginary coherence (iCoh)37. Imaginary coherence is only sensitive to synchronization of two processes that are time-lagged to each other. iCoh is therefore insensitive to volume conduction of common signals and represents true coupling. Using similar parameters as that used for coherence analyses, we derived the imaginary part of coherency between the two signals for non-overlapping time windows.
Statistical analysis
Statistical significance of changes in spectral power between PreAnes and Anes conditions at each frequency (1–300 Hz) for each disease cohort was assessed using two group test of the spectrum, testing the null hypothesis that Anes and Pre-Anes have equal spectra within disease groups38. Since multitaper spectral analysis uses an orthogonal family of tapers (i.e. Slepian sequences) and the analysis was done for non-overlapping windows, obtained tapered spectra are assumed to be statistically independent. We calculated mean group spectra for two conditions (across each cohort), and corresponding Z statistics using asymptotic spectral probability distribution. The 95% confidence intervals were then calculated based on Jackknife estimation of variance as previously described38. To address the issue of multiple comparisons, we note that differences in spectra due to chance are likely to be at isolated frequencies, while neurophysiological differences are likely to occur across contiguous frequency ranges (i.e. α, β). Since spectral estimates at frequencies separated by less than the bandwidth of the multitaper method (4 Hz) are inherently correlated, we rejected the null hypothesis for all candidate frequencies constituting bands whose width is larger than 4 Hz.
Following an approach similar to spectral power comparison, average magnitude-squared coherence between two signals for two conditions were contrasted and statistically compared using two group coherence test38. Subsequent Z-statistics and confidence intervals for a p-value of 0.05 were calculated to assess statistical significance. Correction for multiple comparisons was done in a similar fashion to the spectral analysis.
To assess the statistical significance of differences in group average iCoh between two conditions (PreAnes and Anes), we used permutation testing, pooling together values from all time segments for all subjects (considering that tapers are orthogonal and time windows are non-overlapping, we could reasonably assume that tapered Fourier transforms are interchangeable). At each permutation (n = 10000), a random subset of the cohort was selected for which the labels of the conditions were swapped allowing to create a null distribution of the group difference. Since the difference values at each frequency are bound between −1 and 1, the Fisher Z-transform of the condition difference could be assumed approximately normally distributed under the null hypothesis. The significance of the condition difference was then assessed using P= 0.05 and corrected for multiple comparisons in a similar fashion to the spectral and coherence statistical analysis.
Shapiro-Wilk test was used to explore normality of distributions when comparing peak spectral and coherence frequencies between the two disease groups and within each cohort to explore effects of propofol. Such analysis showed that distribution of the peak frequency values was significantly different from normal distribution (P<0.05). Subsequently, we used Mann-Whitney test to compare frequency of dominant spectral peaks between Parkinson disease and essential tremor groups. We employed Wilcoxon signed-rank test to assess changes in the peak frequencies between Pre-Anes and Anes states separately within each disease group.
Results
Patients demographics and Anesthetic Induction and Maintenance
Nineteen patients were included in this study, including twelve patients with Parkinson disease (3 female, 9 male, average age 65 ±7 years) and seven patients with essential tremor (4 female, 3 male, average age 69 ±6 years). Clinical evaluation of disease severity was done for both disease groups separately prior to the surgery while subjects were off medication. Unified Parkinson disease rating scale (UPDRS), part 3 (motor examination) indicated mean ±SD of 41 ±9 and tremor rating scale for essential tremor indicated 53 ±12 for severity of the symptoms for two groups of patients. Average body weight across the cohort at the time of recording was 79.5 ±19.2 kg. Propofol was administered intravenously with an initial bolus of 0.53 ±0.31 mg/kg, followed by an average continuous infusion rate of 73 ±33 mcg/kg/min.
Propofol induced changes in cortical spectral power and coherence
Propofol-induced loss of consciousness was associated with significant increases in cortical broadband power for frequencies between 2–100 Hz across all three sensorimotor cortical contacts. Such broadband power increase happened in both disease cohorts (Figure 3). These spectral changes were consistent across the duration of the entire examined segments, as illustrated in average TFR maps (Figure 3A and C) including both PreAnes and Anes segments.
Figure 3.
Propofol induced power spectral changes in sensorimotor cortical signals. (A) Group average of time frequency representation of power comparing PreAnes (left) and Anes (right) time segments across three bipolar channels investigated in Parkinson disease. (B) Group average of power spectral density comparing PreAnes (black) and Anes (red) across sensorimotor cortices in Parkinson disease. Blue shade shows significant difference of spectra between two conditions at P =0.05 after correction for multiple comparisons. Inserts indicate shift of the dominant spectral peak from β frequencies to α with propofol anesthesia (C) Similar to (A), shows group average time frequency maps comparing PreAnes and Anes conditions for essential tremor group. (D) Similar to (B) shows average PSDs in essential tremor cohort for two conditions across three cortical signals with significant difference at P =0.05 (after correction for multiple comparisons) highlighted by blue shades. Inserts show that despite local power increase, dominant spectral peak remains in α frequencies in essential tremor group with propofol anesthesia.
In addition to increases in spectral power between 2–100Hz, in both disease groups, the dominant spectral peak (8–35 Hz) in the anesthetized state was in the α band (Figure 3B and D). In Parkinson disease, the median dominant spectral shifts from 24.5 ±2.6 Hz (β range) to 12.8 ±2.3 Hz (p =0.002, paired sample Wilcoxon signed-rank test, Figure 3B, inserts). In essential tremor, the median spectral peak decreased but not significantly from 13.8 ±2.1 Hz to 12.1 ±1.0 Hz (p =0.23, paired sample Wilcoxon signed-rank test, Figure 3D, inserts). While median spectral peaks for essential tremor and Parkinson disease were different preAnes (p=0.0003, Mann-Whitney ranked-sum test), independent samples Mann-Whitney test showed no significant difference in median peak frequency between two groups in the Anes state(P = 0.35).
Analysis of propofol-induced cortical power changes also showed that in the Parkinson disease group, power at frequencies 150–300 Hz significantly decreased at PreCS signal (Figure 3A–B). Such 150–300 Hz power increase was not observed at PostCS and CS signals in Parkinson disease nor any of the cortical signals in the essential tremor cohort.
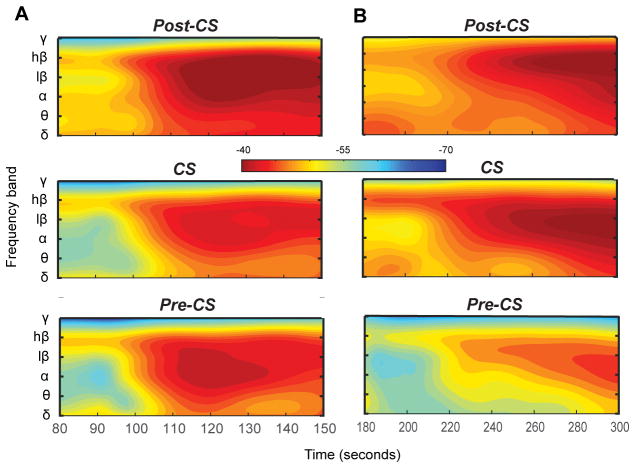
Tracking band power changes over time showed a consistent pattern of relative timing between different frequency bands for 11 (out of 12) subjects in the Parkinson disease group, with high β power (20–35 Hz) increase preceding changes in other frequency bands, especially α/low β (13–20 Hz) band (Figure 4). This orderly pattern was observed irrespective of the dose or the timing of onset of therapeutic effect of propofol. In essential tremor cohort, we observed no consistent pattern of timing in power change across different frequency bands.
Figure 4.
High β power changes precedes changes in other bands in Parkinson disease group with proopfol anesthesia. (A-B) Time frequency representation of relative timing between changes in band power changes for different frequency bands for two representative subjects, highlighting similarity in relative pattern despite difference in timing of the changes. Time stamps are relative to the beginning of the recording (0 seconds) and Propofol was injected at T = 60 seconds.
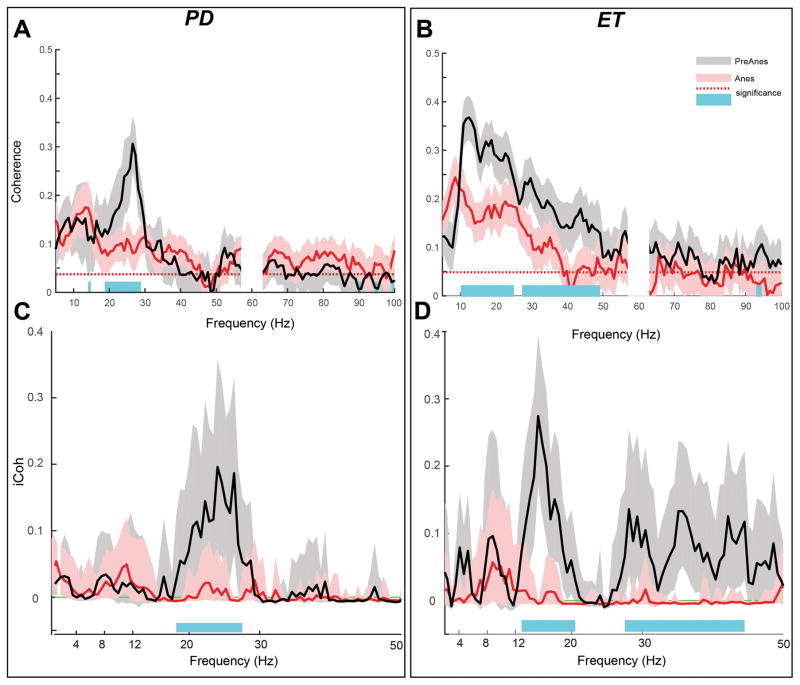
Propofol suppresses sensorimotor cortical coherence
Despite broadband increases in spectral power at each cortical site, sensorimotor cortical coherence decreased with propofol anesthesia (Figure 5A–B). Specifically, sensorimotor coherence significantly decreased in the Parkinson disease cohort in high β frequencies (18–29 Hz) and in the essential tremor cohort across frequencies between 10–48 Hz, including both α and β bands (two group coherence test as described in the methods, P < 0.05). Median peak frequencies for coherence changed from 24.6 ±3.1 Hz to 14.35 ±3.7 Hz in the Parkinson disease group and from 13.4 ±2.0 Hz to 10.4 ±2.8 Hz in the essential tremor group. Wilcoxon signed-rank test revealed that across both cohorts, changes in dominant coherence peak frequency were statistically significant (P=0.002 for Parkinson disease and P=0.028 for essential tremor).
Figure 5.
Suppression of cortical sensorimotor α-β functional connectivity with propofol anesthesia. (A-B) contrasting sensorimotor coherence between PreAnes (black) and Anes (red) conditions, for Parkinson disease and essential tremor groups respectively. Dashed lines indicate 95% confidence level for coherence driven by Jackknife method, indicating that coherence values at PreAnes and Anes conditions are statistically significant. Shades around each average coherence curve show 95% confidence intervals of the mean. Significant differences at P =0.05 level highlighted in blue and corrected for multiple comparisons. (C-D) Imaginary coherence between sensorimotor cortices for PreAnes (black) and Anes (red) for Parkinson disease and essential tremor groups respectively. Dashed lines indicate 95% confidence level for coherence driven by Jackknife method, indicating that coherence values at PreAnes and Anes conditions are statistically significant. Shades around each average coherence curve show 95% confidence intervals of the mean Significant differences at P =0.05 are highlighted with blue and corrected for multiple comparisons.
To confirm that coherence measures do not represent spurious volume conduction, we also evaluated imaginary coherence (iCoh) between cortical signals (Figure 5C–D). In the Parkinson disease cohort, iCoh significantly decreased for frequencies in the high β range (18–29 Hz). In essential tremor group, a significant decrease in iCoh was observed in frequency ranges of (12–20 and 27–44 Hz) including the α and β bands (permutation statistics, as described in the methods, P < 0.05). These results indicate that β coherence suppression with propofol was common across diseases while α coherence suppression with propofol was specific to the essential tremor cohort.
Discussion
Emerging evidence suggests that anesthetics exert their influence by altering functional connectivity within brain networks thereby disrupting cortical integration3,39–41. In this study, propofol-induced changes in sensorimotor cortical activity in 19 movement disorder patients, demonstrates that propofol anesthesia is associated with local power increase in α and β oscillations along with concurrent reduction in inter-cortical sensorimotor coupling at these frequencies. Such a scenario emphasizes that functional disconnection is likely a product of altered phase-based connectivity, regardless of changes in the amplitudes of the signals. It is therefore likely that propofol induces a functional disconnection between cortical areas along with increases in autonomous activity within these regions. This pattern occurs similarly across diseases evaluated, suggesting these may be generalizable effects of propofol. Swann and colleagues who examined subcortical-motor cortex connectivity in a mixed group of patients with movement disorders30 also observed propofol-induced reduction in subcortical-motor cortex coupling specific to β frequencies. Our current study further suggests that propofol administration results in the reduction of both inter-cortical and cortical-subcortical functional connectivity.
Identifying common signatures of anesthetic interaction with electrophysiological activity is a fundamental step towards understanding the anesthetic-induced unconsciousness42. A recent non-human primate study on the effects of ketamine in sensorimotor cortical activity, similarly found functional disconnection between the sensory and motor cortices at β frequencies, with concurrent β power suppression isolated to the sensory cortex43. Despite some differences which may at least be due to differences in anesthetic mechanisms of ketamine and propofol, a common theme emerges of sensorimotor disconnection in the anesthetized state.
Dissimilarities noted in the baseline sensorimotor α coherence observed between the two disease groups may be due to the difference in the disease specific pathophysiological mechanisms. Studies comparing cortical activity between Parkinson disease and essential tremor have shown that dominant spectral peaks for these disease states respectively reside within the β and α range44. The stronger α peak in essential tremor has been suggested to reflect pathological entrainment of basal ganglia-thalamo-cortical network at tremor and double tremor frequencies 45,46
Propofol-induced loss of consciousness results in dominance of local α activity in sensorimotor cortices
Propofol-induced enhancement of α oscillations are well described47,48. Our results also confirm that propofol amplifies α band oscillations across sensorimotor cortices. Although baseline dominant spectral peaks differed across groups, propofol eliminated this difference, such that both disease groups had dominant α band peaks after propofol administration. This propofol-induced α dominance is interesting in the context of movement suppression since α has been implicated to functionally inhibit unneeded neural networks within a hemisphere23,27. These oscillations are suggested to regulate the “akinetic” state of the basal ganglia-thalamocortical motor network27–29, possibly inhibiting movement through modulating pyramidal tract neuronal firing rate24.
Despite reduced β synchrony between cortices local β power increases with propofol
Discordances have been noted between simultaneously recorded ECoG and EEG signals in non-human primates in which pharmacological manipulation of brain activity has been shown to modulate EEG signals independent of changes observed via intracortical recordings49. This suggests that ECoG and EEGs can be decoupled due to a variety of electrophysiological and anatomical variables17,49. Although we consistently observed increases in local cortical β power in all 19 subjects included in this study, this is at odds with most of the EEG literature suggesting cortical β power is suppressed with propofol, apart from transient “paradoxical” excitation in β power that has been associated with a hyperkinetic state8–10. Using intracranial EEG recordings,50 Verdonck and colleagues found a similar 1–30 Hz power increase in motor cortex with propofol. Moreover, a recent study of propofol-induced loss of consciousness in non-human primates showed that upon the onset of loss of consciousness, inter-cortical coherence β diminished while these oscillations remained locally coherent within sensorimotor cortices17. Interestingly, Swann and colleagues, using ECoG recordings during DBS surgery in similar groups of patients with movement disorders, did not report β power change across their cohort30. However their subject-level analysis show β power increase at least for some subjects in their cohort, consistent with our current findings.
Our results are unlikely to be explained by paradoxical excitation which is behaviorally manifested by disinhibition of movements, restlessness, agitation, talkativeness, small, spontaneous muscle movements or dystonic or choreiform movements of the arms and legs during induction51,52. Paradoxical excitation is created by low doses of anesthetics and is marked by an increase in β band activity and a decrease in activity of lower frequencies (α and θ) observed in the EEG signal53. A cortical model of this phenomenon attributes the generation of β-band activity at low doses of propofol to cortical dynamics involving the interaction of pyramidal neurons with inhibitory interneurons54. Since cortical pyramidal cells are thought to be a main source of the EEG signals, at the population level, this interaction manifests as an increase in the spiking frequency of pyramidal neurons in the β range9,54. ECoG does not face the low-pass filtering effects of skull and skin present in the EEG. Moreover, the uniformity of findings across subjects, across cortical contacts, and across doses of propofol with loss of verbal responsiveness and spontaneous movements in each patient suggests the current findings are not spurious, due to artifact, or due to the paradoxical excitation11 but rather true physiological phenomenon.
Alternative interpretation of elevated local β activity
β oscillations play a key role in motor network physiology, controlling information coding capacity across the motor loops of the basal ganglia-thalamocortical circuit24 and exhibit strong movement-related modulation throughout the motor network25,55,56. Periods of elevated β power are associated with slowing of spontaneous movement and increased corrective responses to postural perturbation suggesting that β actively stabilizes the current motor set57. This function becomes pathologically exaggerated in Parkinson disease, resulting in rigidity and bradykinesia58,59. However, even in Parkinson disease, the level of β activity is dynamic, likely fluctuating with moment-to-moment variations in dopaminergic activity in response to salient internal and external cues. In essential tremor, comparable levels of resting state β activity have been observed at sensorimotor cortices with no significant difference relative to Parkinson disease44,60. These findings suggest that aberrant β activity within the cortical sensorimotor region is likely a common manifestation between two different pathologies. Our variable result of propofol-induced β power increase in cortical activity may, therefore, in part be due to the underlying pathophysiology of Parkinson disease and essential tremor in the current patient population.
Potential mechanisms for propofol induced local α-β power increase and inter-regional disconnection
It remains unclear if propofol-induced increases in local cortical sensorimotor α-β power and suppression of inter-regional β synchrony are a consequence of alterations in corticocortical versus subcortical-cortical connectivity. Plausible explanation for these findings could include: 1) an elevation of the thalamocortical network activity that drives α oscillations, resulting in α dominant spectral peaks across subjects and 2) the reduction of sensorimotor cortical connectivity within the β band
Network level studies of anesthesia suggest a reduction in inter-areal connectivity from frontal to parietal cortices (feedback projections), but preserves parietal to frontal connectivity14,15,20,61. It has been suggested that feedforward projections represent incoming sensory data whereas feedback projections play a modulating role in the information selection62. Therefore selective inhibition of top-down feedback projection during loss of consciousness, could be interpreted as decreased higher-order cognitive processing, while maintaining lower-order sensory processing20. Consistent with this theory, Schroeder and colleagues provided evidence of disruption in top-down sensorimotor integration under ketamine anesthesia in a non-human primate model43. Although in the current study, we have not directly investigated the changes in effective connectivity nor encoding of sensory information from our recordings, our findings on functional disconnection of sensorimotor cortices by induction of anesthesia further supports that disruption of fronto-parietal information transfer is likely to be a common proxy of anesthetic induced unconsciousness.
Limitations
The study of invasive electrophysiological activity in the human brain is limited to neurosurgical patients. In some cases, recordings from patients with other non-movement neurological conditions (e.g., epilepsy) are used for comparison instead of control groups46. The discrepancy of results between ECoG and EEG recordings are suggested to be intensified during transitional states as also observed by Ishizawa and colleagues17. Therefore meticulous monitoring of subjects during induction of anesthesia is essential to understanding the microscale anesthetic-related changes in neuronal activity. Given the fact that patients included in this study are not intubated and depth of anesthesia is being assessed only using standard anesthesia scales, the precision of timing with which loss of consciousness is assessed might not be optimal. Future studies should benefit from changing the induction protocol so that monitoring for induction of anesthesia could be preferably done using a target-controlled infusion to have an approximately stable serum concentration during the anesthetic phase. In particular, one must account for effects of variable dosing as well as variable cardiac output and blood volume that can lead to a substantial difference in circulation time and anesthetic induction across subjects. Finally, comparison of induction and emergence can provide greater insight into the causal relationships in these network signals.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Biomedical Imaging and Bioengineering [K23 EB014326], National Institutes of Neurological Disorders and Stroke [R01NS097782] and philanthropic support from Casa Colina Centers for Rehabilitation. MM also was supported by postdoctoral fellowship from American Parkinson’s disease association (APDA, NY, USA)
This work was supported by the National Institutes of Biomedical Imaging and Bioengineering [K23 EB014326], National Institutes of Neurological Disorders and Stroke [R01NS097782] and philanthropic support from Casa Colina Centers for Rehabilitation. MM also was supported by a postdoctoral fellowship from American Parkinson’s disease association (APDA, NY, USA). Authors would like to thank patients who consented to participate in this study without whom recording of local field potentials would not be possible.
Footnotes
Conflict of Interests: Authors report no competing interests
Competing Interests
The authors declare no competing interests.
References
- 1.Bai D, Pennefather PS, MacDonald JF, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABA(A) receptors. J Neurosci. 1999;19:10635–46. doi: 10.1523/JNEUROSCI.19-24-10635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–28. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, Anderson WS, Hochberg LR, Cash SS, Brown EN, Purdon PL. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–86. doi: 10.1073/pnas.1210907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrouff J, Perlbarg V, Boly M, Marrelec G, Boveroux P, Vanhaudenhuyse A, Bruno MA, Laureys S, Phillips C, Pélégrini-Issac M, Maquet P, Benali H. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57:198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–80. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- 7.Feshchenko VA, Veselis RA, Reinsel RA. Propofol-induced alpha rhythm. Neuropsychobiology. 2004;50:257–66. doi: 10.1159/000079981. [DOI] [PubMed] [Google Scholar]
- 8.Mukamel EA, Pirondini E, Babadi B, Wong KF, Pierce ET, Harrell PG, Walsh JL, Salazar-Gomez AF, Cash SS, Eskandar EN, Weiner VS, Brown EN, Purdon PL. A transition in brain state during propofol-induced unconsciousness. J Neurosci. 2014;34:839–45. doi: 10.1523/JNEUROSCI.5813-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KF, Salazar-Gomez AF, Harrell PG, Sampson AL, Cimenser A, Ching S, Kopell NJ, Tavares-Stoeckel C, Habeeb K, Merhar R, Brown EN. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–51. doi: 10.1073/pnas.1221180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical Electroencephalography for Anesthesiologists Part I: Background and Basic Signatures. Anesthesiology. 2015;123:937–60. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ching S, Brown EN. Modeling the dynamical effects of anesthesia on brain circuits. Curr Opin Neurobiol. 2014;25:116–22. doi: 10.1016/j.conb.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ching SN, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:22665–70. doi: 10.1073/pnas.1017069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijayan S, Ching S, Purdon PL, Brown EN, Kopell NJ. Thalamocortical mechanisms for the anteriorization of alpha rhythms during propofol-induced unconsciousness. J Neurosci. 2013;33:11070–5. doi: 10.1523/JNEUROSCI.5670-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boly M, Moran R, Murphy M, Boveroux P, Bruno MA, Noirhomme Q, Ledoux D, Bonhomme V, Brichant JF, Tononi G, Laureys S, Friston K. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–90. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez F, Phillips C, Soddu A, Boly M, Boveroux P, Vanhaudenhuyse A, Bruno MA, Gosseries O, Bonhomme V, Laureys S, Noirhomme Q. Changes in effective connectivity by propofol sedation. PLoS One. 2013;8:e71370. doi: 10.1371/journal.pone.0071370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaskell AL, Hight DF, Winders J, Tran G, Defresne A, Bonhomme V, Raz A, Sleigh JW, Sanders RD. Frontal alpha-delta EEG does not preclude volitional response during anaesthesia: prospective cohort study of the isolated forearm technique. BJA Br J Anaesth. 2017;126:371–2. doi: 10.1093/bja/aex170. [DOI] [PubMed] [Google Scholar]
- 17.Ishizawa XY, Ahmed OJ, Patel SR, Gale XJT, Sierra-mercado D, Brown EN, Eskandar EN. Dynamics of Propofol-Induced Loss of Consciousness Across Primate Neocortex. J Neurosci. 2016;36:7718–26. doi: 10.1523/JNEUROSCI.4577-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA. Disruption of Frontal–Parietal Communication by Ketamine, Propofol, and Sevoflurane. Anesthesiology. 2013;118:1264–75. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Untergehrer G, Jordan D, Kochs EF, Ilg R, Schneider G. Fronto-Parietal Connectivity Is a Non-Static Phenomenon with Characteristic Changes during Unconsciousness. PLoS One Edited by Ward LM. 2014;9:e87498. doi: 10.1371/journal.pone.0087498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan D, Ilg R, Riedl V, Schorer A, Grimberg S, Neufang S, Omerovic A, Berger S, Untergehrer G, Preibisch C, Schulz E, Schuster T, Schröter M, Spoormaker V, Zimmer C, Hemmer B, Wohlschläger A, Kochs EF, Schneider G. Simultaneous Electroencephalographic and Functional Magnetic Resonance Imaging Indicate Impaired Cortical Top–Down Processing in Association with Anesthetic-induced Unconsciousness. Anesthesiology. 2013;119:1031–42. doi: 10.1097/ALN.0b013e3182a7ca92. [DOI] [PubMed] [Google Scholar]
- 21.Nourski KV, Banks MI, Steinschneider M, Rhone AE, Kawasaki H, Mueller RN, Todd MM, Howard MA. Electrocorticographic delineation of human auditory cortical fields based on effects of propofol anesthesia. Neuroimage. 2017;152:78–93. doi: 10.1016/j.neuroimage.2017.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engel AK, Fries P. Beta-band oscillations-signalling the status quo? 2010;20:156–65. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijk BCM, van Beek PJ, Daffertshofer A. Neural synchrony within the motor system: what have we learned so far? Front Hum Neurosci. 2012;6:1–15. doi: 10.3389/fnhum.2012.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohara S, Mima T, Baba K, Ikeda A, Kunieda T, Matsumoto R, Yamamoto J, Matsuhashi M, Nagamine T, Hirasawa K, Hori T, Mihara T, Hashimoto N, Salenius S, Shibasaki H. Increased synchronization of cortical oscillatory activities between human supplementary motor and primary sensorimotor areas during voluntary movements. J Neurosci. 2001;21:9377–86. doi: 10.1523/JNEUROSCI.21-23-09377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arce-McShane FI, Ross CF, Takahashi K, Sessle BJ, Hatsopoulos NG. Primary motor and sensory cortical areas communicate via spatiotemporally coordinated networks at multiple frequencies. Proc Natl Acad Sci. 2016;113:5083–8. doi: 10.1073/pnas.1600788113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niedermeyer E. Alpha rhythms as physiological and abnormal phenomena. Int J Psychophysiol. 1997;26:31–49. doi: 10.1016/s0167-8760(97)00754-x. [DOI] [PubMed] [Google Scholar]
- 28.Brittain J-SS, Sharott A, Brown P. The highs and lows of beta activity in cortico-basal ganglia loops. Eur J Neurosci. 2014;39:1951–9. doi: 10.1111/ejn.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci. 2004;24:9240–3. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swann NC, Hemptinne C, de Maher RB, Stapleton CA, Meng L, Gelb AW, Starr PA. Motor System Interactions in the Beta Band Decrease during Loss of Consciousness. J Cogn Neurosci. 2015:1–12. doi: 10.1162/jocn_a_00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsiokos C, Hu X, Pouratian N. 200–300Hz movement modulated oscillations in the internal globus pallidus of patients with Parkinson’s Disease. Neurobiol Dis. 2013;54:464–74. doi: 10.1016/j.nbd.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsiokos C, Malekmohammadi M, Au Yong N, Pouratian N. Pallidal low β-low γ phase-amplitude coupling inversely correlates with Parkinson disease symptoms. Clin Neurophysiol. 2017 doi: 10.1016/j.clinph.2017.08.001. [DOI] [PMC free article] [PubMed]
- 33.Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. 1990;10:244–51. [PubMed] [Google Scholar]
- 34.Oostenveld R, Fries P, Maris E, Schoffelen J-M, Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data, FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput Intell Neurosci Comput Intell Neurosci 2010. 2011;2011:e156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: a platform for analyzing neural signals. J Neurosci Methods. 2010;192:146–51. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70:1055–96. [Google Scholar]
- 37.Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115:2292–307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Bokil H, Purpura K, Schoffelen JM, Thomson D, Mitra P. Comparing spectra and coherences for groups of unequal size. J Neurosci Methods. 2007;159:337–45. doi: 10.1016/j.jneumeth.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mashour GA. Consciousness unbound: toward a paradigm of general anesthesia. Anesthesiology. 2004;100:428–33. doi: 10.1097/00000542-200402000-00035. [DOI] [PubMed] [Google Scholar]
- 41.Sarasso S, Rosanova M, Casali AG, Casarotto S, Fecchio M, Boly M, Gosseries O, Tononi G, Laureys S, Massimini M. Quantifying cortical EEG responses to TMS in (un)consciousness. Clin EEG Neurosci. 2014;45:40–9. doi: 10.1177/1550059413513723. [DOI] [PubMed] [Google Scholar]
- 42.Hudetz AG, Mashour GA. Disconnecting Consciousness. Anesth Analg. 2016;123:1228–40. doi: 10.1213/ANE.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder KE, Irwin ZT, Gaidica M, Nicole Bentley J, Patil PG, Mashour GA, Chestek CA. Disruption of corticocortical information transfer during ketamine anesthesia in the primate brain. Neuroimage. 2016;134:459–65. doi: 10.1016/j.neuroimage.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crowell AL, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Shimamoto S, Lim DA, Starr PA. Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain. 2012;135:615–30. doi: 10.1093/brain/awr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- 46.Kondylis ED, Randazzo MJ, Alhourani A, Lipski WJ, Wozny TA, Pandya Y, Ghuman AS, Turner RS, Crammond DJ, Richardson RM. Movement-related dynamics of cortical oscillations in Parkinson’s disease and essential tremor. Brain. 2016;139:2211–23. doi: 10.1093/brain/aww144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breshears JD, Roland JL, Sharma M, Gaona CM, Freudenburg ZV, Tempelhoff R, Avidan MS, Leuthardt EC. Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia. Proc Natl Acad Sci U S A. 2010;107:21170–5. doi: 10.1073/pnas.1011949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanrahan SJ, Greger B, Parker RA, Ogura T, Obara S, Egan TD, House PA. The effects of propofol on local field potential spectra, action potential firing rate, and their temporal relationship in humans and felines. Front Hum Neurosci. 2013;7:136. doi: 10.3389/fnhum.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musall S, Pföstl V, Von Rauch A, Logothetis NK, Whittingstall K. Effects of neural synchrony on surface EEG. Cereb Cortex. 2014;24:1045–53. doi: 10.1093/cercor/bhs389. [DOI] [PubMed] [Google Scholar]
- 50.Verdonck O, Reed SJ, Hall J, Gotman J, Plourde G. The sensory thalamus and cerebral motor cortex are affected concurrently during induction of anesthesia with propofol: a case series with intracranial electroencephalogram recordings. Can J Anesth Can d’anesthésie. 2014;61:254–62. doi: 10.1007/s12630-013-0100-y. [DOI] [PubMed] [Google Scholar]
- 51.Fulton SA, Mullen KD. Completion of upper endoscopic procedures despite paradoxical reaction to midazolam: a role for flumazenil? Am J Gastroenterol. 2000;95:809–11. doi: 10.1111/j.1572-0241.2000.01866.x. [DOI] [PubMed] [Google Scholar]
- 52.Sneyd JR. Excitatory events associated with propofol anaesthesia: a review. J R Soc Med. 1992;85:288–91. doi: 10.1177/014107689208500516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gugino LD, Chabot RJ, Prichep LS, John ER, Formanek V, Aglio LS. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. Br J Anaesth. 2001;87:421–8. doi: 10.1093/bja/87.3.421. [DOI] [PubMed] [Google Scholar]
- 54.McCarthy MM, Brown EN, Kopell N. Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation. J Neurosci. 2008;28:13488–504. doi: 10.1523/JNEUROSCI.3536-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gross J, Pollok B, Dirks M, Timmermann L, Butz M, Schnitzler A. Task-dependent oscillations during unimanual and bimanual movements in the human primary motor cortex and SMA studied with magnetoencephalography. Neuroimage. 2005;26:91–8. doi: 10.1016/j.neuroimage.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 56.Houweling S, Daffertshofer A, Dijk BW, van Beek PJ. Neural changes induced by learning a challenging perceptual-motor task. Neuroimage. 2008;41:1395–407. doi: 10.1016/j.neuroimage.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Little S, Brown P. The functional role of beta oscillations in Parkinson’s disease. Park Relat Disord. 2014;20(Suppl 1):S44–8. doi: 10.1016/S1353-8020(13)70013-0. [DOI] [PubMed] [Google Scholar]
- 58.Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider G-HH, Brown P, Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A, Schneider G-HH, Brown P, Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider G-HH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–7. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Little S, Pogosyan A, Kuhn AA, Brown P. beta band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp Neurol. 2012;236:383–8. doi: 10.1016/j.expneurol.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowland NC, Hemptinne C, De Swann NC, Qasim S, Miocinovic S, Ostrem JL, Knight RT, Starr PA. Task-related activity in sensorimotor cortex in Parkinson’s disease and essential tremor: changes in beta and gamma bands. Front Hum Neurosci. 2015;9:512. doi: 10.3389/fnhum.2015.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ku S-W, Lee U, Noh G-J, Jun I-G, Mashour GA. Preferential Inhibition of Frontal-to-Parietal Feedback Connectivity Is a Neurophysiologic Correlate of General Anesthesia in Surgical Patients. PLoS One Edited by Ward LM. 2011;6:e25155. doi: 10.1371/journal.pone.0025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: Shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.