Abstract
BACKGROUND:
Food restriction has been demonstrated to increase the alertness in different species and to increase the levels of the wake-promoting neurotransmitter orexin. We hypothesized that diurnal intermittent fasting (DIF) increases orexin-A levels during fasting. Therefore, we conducted this study to assess the effects of DIF, during the month of Ramadan, on orexin, while controlling for lifestyle changes that may accompany Ramadan such as sleep duration, bedtime and wake time, energy expenditure, light exposure, and food.
METHODS:
Eight young healthy volunteers (mean age, 25.4 ± 3.5 years) reported to the laboratory on three occasions: (1) 4 weeks before Ramadan while performing DIF for 1 week outside the month of Ramadan (fasting outside Ramadan); (2) 1 week before Ramadan (nonfasting baseline) (BL); and (3) during the 2nd week of Ramadan while performing DIF (Ramadan). Plasma levels of orexin-A were measured using an enzyme immunoassay five times at 22:00, 02:00, 04:00, 06:00, and 11:00. Caloric intake, light exposure, and sleep schedule were maintained during the participants’ stays in the laboratory in the three study periods.
RESULTS:
Orexin-A levels increased in the daytime during fasting and decreased at night compared to BL. The differences in orexin-A levels between DIF and BL were significant at 06:00, 11:00, 22:00, and 02:00.
CONCLUSIONS:
DIF increases orexin-A levels in the plasma during fasting hours. This finding supports findings from animal studies showing that fasting increases alertness.
Keywords: Hypocretin, intermittent fasting, orexin, Ramadan, sleep, vigilance
The relationship between diurnal intermittent fasting (DIF) (represented mainly by fasting during Ramadan) and sleepiness is an interesting topic with contradicting results reported in previous studies.[1] In general, food restriction has been demonstrated to increase alertness in different species.[2] However, this topic is not well studied with respect to the DIF. Several studies have assessed daytime sleepiness during DIF during Ramadan both subjectively[3,4,5,6,7,8,9,10] and objectively.[6,8,9] These studies reported contradictory results, which may be due to differences in the methods used to assess sleepiness, different populations and cultures, and lack of control for potential confounders that may accompany DIF such as light exposure, meal composition, and wake time and bedtime, which could potentially affect daytime alertness.[1] Therefore, it would be a good idea to measure the effects of DIF on levels of natural and endogenous markers of sleepiness and wakefulness, such as neurotransmitters. Sleep and wakefulness are controlled by a complex system that involves several sleep-promoting (inhibitory) and wake-promoting (excitatory) neurotransmitters.[11] A recently discovered hypothalamic neuropeptide that can be measured in blood plasma is orexin-A. Orexin-A (hypocretin-1) is a wake-promoting (excitatory) neurotransmitter secreted by the hypothalamus. There are two types of orexin, orexin-A and orexin-B (hypocretin-1 and hypocretin-2). Both are excitatory neuropeptides; however, orexin-A is more stable and more lipophilic than is orexin-B and can be measured in the cerebrospinal fluid (CSF) and plasma.[12]
Orexin-A is usually affected by nutritional status and food intake.[13] Fasting has been shown to upregulate orexin gene expression in animal models.[2] Moreover, it has been shown that direct injection of orexin into the laterodorsal tegmental nucleus in animal models increased wake time.[14] However, as experimental fasting in animal models entails fasting that is usually more prolonged than the DIF practiced during Ramadan, findings extrapolated from animal studies cannot be extrapolated to Ramadan fasting.
Usually, orexin-A levels are measured in the CSF; however, plasma levels of orexin-A can be measured with good accuracy.[15] A strong correlation between CSF and plasma concentrations of orexin-A has been reported in healthy human subjects, as well as in mice.[16] It is postulated that the highly lipophilic orexin-A (but not orexin-B) rapidly enters the brain from the blood through a nonsaturable mechanism.[13] Plasma levels of orexin-A have been used to assess the response of sleepiness to positive airway pressure therapy in patients with obstructive sleep apnea (OSA).[15] Moreover, it has been shown that plasma levels of orexin-A have a significant linear correlation with daytime sleepiness in patients with OSA.[17] It has been hypothesized that the increased plasma levels of orexin-A in patients with OSA with daytime sleepiness indicate a physical response to increase wakefulness and counteract the effects of sleepiness.[17] Therefore, orexin-A appears to be a good marker to assess the sleepiness during DIF.
To the best of our knowledge, no study has assessed orexin-A levels in humans during DIF so far. Therefore, we conducted the present study to assess the effects of DIF on orexin-A levels in healthy human subjects. As orexin-A levels can be affected by light exposure,[18] sleep pattern,[18] and exercise,[19] we controlled for lifestyle changes that may accompany Ramadan such as sleep duration, bedtime and wake time, energy expenditure, light exposure, and food intake.
Methods
Subjects
Eight healthy, nonsmoker male volunteers between the ages of 20 and 30 years were included in the present study. Volunteers were recruited through advertising on bulletin boards in the local hospital and university. The project was approved by the Institutional Review Board of the College of Medicine, King Saud University, and informed consent was obtained. Inclusion criteria included not being on any medications or special diet, no history of smoking, alcohol drinking, no night shift work, and not being on vacation during the study period. Exclusion criteria included patients with documented medical, psychiatric, or sleep disorders. In addition, all participants were assessed by one of the authors to make sure that they had no medical problems or sleep disorders. Moreover, before enrollment, participants’ sleep patterns were assessed via actigraphy (Philips/Respironics, Inc., Murrysville, PA, USA) (1 week of continuous monitoring),[20] and their circadian patterns were assessed via a validated Arabic version of the reduced Horne and Ostberg's Morningness–Eveningness questionnaire.[21,22] All participants had the morning chronotypes and a regular wake/sleep pattern. A regular sleep pattern was defined as <1 h of daily variability in the bedtime and wake time.[23] The participants’ work hours were from 07:30 to 16:30 before Ramadan and from 10:00 to 15:00 during Ramadan.
Study protocol
The present study is a repeated measures study. For details about study design, we refer the reader to previous studies assessing neurohormonal changes during Ramadan.[8,24]
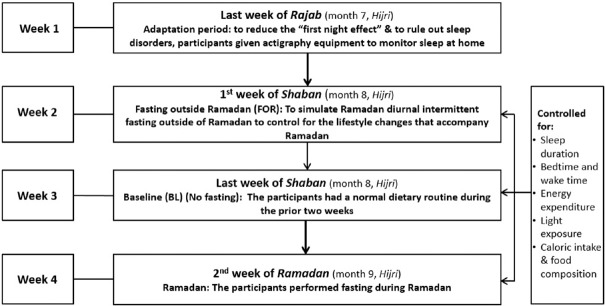
The present study was conducted over the period starting the last week of Rajab (month 7, Hijri), the first and last weeks of Shaban (month 8, Hijri), and the 2nd week of Ramadan (month 9, Hijri) in the Hijri year 1437 (corresponding to May 1–June 21, 2016). The Islamic Hijri calendar follows the lunar system. Each Hijri year contains 354 days, or 11 days fewer than the Gregorian year. As a result, the month of Ramadan occurs 11 days earlier every Gregorian year.[25] While at home during the study periods, sleep was monitored continuously using actigraphy.
Study periods
Fasting outside Ramadan
Participants performed DIF similar to the Islamic intermittent fasting during Ramadan (i.e., abstinence from food and drinks between dawn and sunset) during the 1st week of the Shaban (month 8, Hijri) and were admitted on the last day of the week. This intermittent fasting was introduced to simulate Islamic intermittent fasting outside of Ramadan (FOR) to control for the lifestyle changes and eating habits that accompany Ramadan and may affect sleep and sleepiness such as delays in the start time of work.[1]
Baseline (nonfasting; baseline)
This admission occurred during the last week of Shaban (month 8, Hijri). During the 2 weeks that preceded this admission, the participants had a normal dietary routine.
Ramadan
This visit occurred during the last day of the 2nd week of Ramadan (month 9, Hijri).
Figure 1 shows the study protocol. An adaptation visit was done during the last week of Rajab (month 7, Hijri) to adapt to the sleeping environment and equipment used and to undergo an overnight sleep study (not included in the analysis) to minimize the “ first night effect”[26] and to rule out sleep disorders. Body weight was measured at the beginning of the study and at the end of each of the study periods. Volunteers were asked to wear actigraphy equipment while at home to objectively assess their sleep schedule (Philips/Respironics, Inc.).[20] The actigraphy equipment was worn on the nondominant wrist.[20]
Figure 1.
Study protocol
For orexin-A measurements, the participants were admitted to the sleep laboratory three times (baseline [BL], FOR, and Ramadan). During each admission, participants spent 24 h at the laboratory and underwent overnight polysomnography (PSG). Participants were admitted to the laboratory at approximately 18:00. During the laboratory stay, the participants received meals with a fixed caloric content (35 Kcal/kg/24 h), and fixed proportions of carbohydrates, fats, and proteins. In addition, light exposure was controlled during the three study periods (baseline [BL], FOR, and Ramadan) to minimize its effect on sleepiness, circadian rhythm, and orexin-A levels. From 18:00 until bedtime, the light intensity was maintained at 50 lux. During sleep, all lights were turned off and the light intensity was <1 lux. During Suhur time, light intensity was maintained at <30 lux.[27] The light intensity was measured using SpectralStar Light Meter LX-1 (Japan).
Diurnal intermittent fasting protocol
We used the same intermittent fasting protocol described in previous studies.[8,23,24] During the fasting periods (FOR and Ramadan), the participants were asked to fast from dawn to sunset.
Time of meals in the laboratory
Meal timing was monitored during stays in the sleep laboratory. During BL, the participants had three meals: dinner at 20:00, breakfast at 07:15, and lunch at 12:00. During FOR and Ramadan, the participants had the following meals: breakfast (at sunset, between 18:30 and 18:45), dinner at 21:00, and Suhur (predawn meal) before dawn (between 02:55 and 03:10) to account for the changes in dawn and sunset times.
Timing of blood specimen collection
On the day of admission to the laboratory, an intravenous cannula and a peripheral intravenous line were fixed in the antecubital vein for blood withdrawal. Blood samples were collected at 22:00, 02:00, 04:00, 06:00, and 11:00. The samples were immediately centrifuged at 4°C and stored at −70°C.
Polysomnography
A standard level I attended PSG recording was performed during sleep while in the laboratory during the three study periods −BL, FOR, and Ramadan. The details have been described in previous publications.[8] Alice 6 diagnostic equipment was used for data acquisition (Philips/Respironics, Inc.). The arousal index, which is a measure of sleep fragmentation, was defined as the number of arousals per hour of sleep. The number of “stage shifts” was defined as the total number of changes in sleep stage from lights out to lights on. Scoring was performed according to established scoring criteria.[28]
Sleep and wake pattern and energy expenditure in the laboratory
Daytime naps were not allowed while in the laboratory. During BL, bedtime was at 23:00 and rise time was at 07:00. During FOR and Ramadan, bedtime was 23:00. During Ramadan, the participants had a Suhur meal at approximately 03:00, and the participants went back to sleep at 03:45 until 07:45. During FOR, the participants had a Suhur meal at 03:10 (to account for the shift in the dawn prayer time), and the participants went back to sleep at 03:55, and slept until 07:45.
As exercise may affect the level of orexin-A, energy expenditure was monitored while the participants were in the laboratory using a SenseWear Pro Armband™ (Body Media, Pittsburgh, PA, USA). The technical characteristics of the armband have been previously described.[29,30] In brief, the armband is a small portable sensor worn on the triceps of the right arm and provides information regarding the total energy expenditure and total sleep time.[31] The accuracy of the armband has been validated against double-labeled water and metabolic carts.[31,32,33]
Determination of orexin-A levels
Plasma orexin-A levels were determined using a validated enzyme immunoassay system designed to measure orexin-A in plasma (Phoenix Pharmaceuticals, Burlingame, CA, USA).[34] The assay sensitivity for orexin-A was 0.22 ng/ml, and the specificity for detecting orexin-A was 100%. The intra- and inter-assay coefficients of variation were <5% and <14%, respectively. The measurement procedure was carried out according to the manufacturer's instructions.
Statistical analysis
Data are expressed in the text as the mean ± standard deviation. Energy expenditure was expressed as metabolic equivalents (METs). The average METs were calculated to obtain an overall hourly average for each day.
Comparisons of the BL, FOR, and Ramadan fasting groups were performed using a repeated measures analysis of variance (ANOVA). When the normality test failed, Friedman's repeated measures ANOVA on ranks test was used. Post hoc analyses were performed to determine the significant differences between individual groups. The results were considered statistically significant when they had P < 0.05. The data were analyzed using Statistical Package for Social Sciences software (versions 22; IBM Corporation, Armonk, NY, USA).
Results
Study group characteristics
The mean age of the participants was 25.4 ± 3.5 years and the mean body mass index was 24.4 ± 3.8 kg/m2. No changes occurred in body weight during the three study periods (71.2 ± 7.9 kg, 70.5 ± 6.6 kg, and 69.6 ± 10.8 kg for BL, FOR, and Ramadan, respectively, P = 0.3). In addition, no significant changes were detected in serum glucose measurements, while in the laboratory, measured at 15:30, in the three study periods (5.9 ± 0.6, 5.8 ± 0.5, and 5.5 ± 0.7 mmol/L during BL, FOR, and Ramadan, respectively).
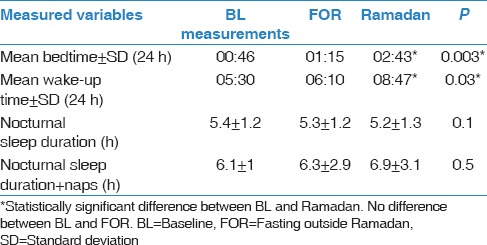
Sleep/wake schedule while sleeping at home
While sleeping at home, there were no significant differences in bedtime and wake time between BL and FOR [Table 1]. However, a significant delay in bedtime and wake-up time was observed during Ramadan, which supports previous studies’ observations that lifestyle changes do occur during Ramadan.[1] No significant differences were detected between the nocturnal sleep duration and total sleep duration (nocturnal sleep and naps) during the three study periods.
Table 1.
Sleep/wake schedule while at home during the study periods
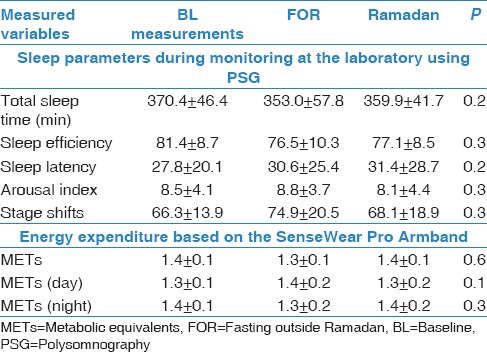
Sleep parameters and energy expenditure during admission to the laboratory
No differences were detected in measured sleep parameters during the three study periods while admitted to the sleep laboratory [Table 2]. No significant changes were found in energy expenditure during the three monitoring periods.
Table 2.
Sleep parameters and energy expenditure during admission to the laboratory in the three study periods
Orexin-A concentrations during the study periods
The pattern of orexin concentrations during BL, FOR, and Ramadan is shown in Figure 2. Noticeably, during the fasting periods, orexin-A levels were lower at night and higher during daytime compared to during BL. This observation was more obvious during FOR. The difference between BL and both FOR and Ramadan was statistically significant at 06:00 (1.6 ± 0.7, 2.5 ± 0.8, and 2.4 ± 0.8, respectively) and at 11:00 (1.6 ± 0.3, 2.7 ± 0.5, and 2.4 ± 0.5, respectively). Moreover, the difference between BL and FOR was statistically significant at 22:00 (2.4 ± 0.9 vs. 1.6 ± 0.6, respectively) and at 02:00 (2.0 ± 0.3 vs. 1.6 ± 0.8, respectively). Although there was a clear trend of higher orexin-A levels at 04:00 during Ramadan and FOR compared to BL, the difference was not statistically significant [Figure 2].
Figure 2.
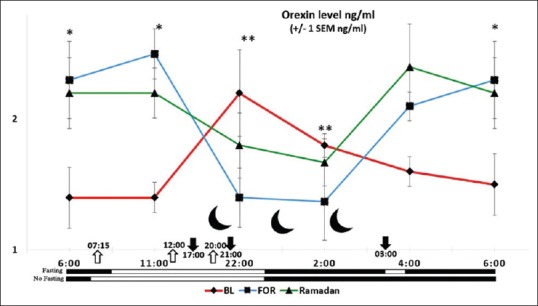
Circadian pattern of plasma orexin-A concentrations in eight healthy male volunteers before and during Ramadan daytime fasting. Each point represents the mean and standard deviation of the eight subjects. Values at baseline (…♦…) (red line), baseline fasting before Ramadan (---◾---) (blue line), and Ramadan (-▴-) (green line) are presented. The dark-headed arrows indicate the approximate meal time during fasting (Ramadan and fasting outside Ramadan), and the white-headed arrows indicate the meal time during baseline. (◻) indicates wakefulness and (◼) indicates sleep. *The difference between both Ramadan and fasting outside of Ramadan and baseline measurements is significant (P < 0.05). **The difference between fasting outside Ramadan and baseline is significant (P < 0.05)
Discussion
Food restriction has been shown to increase wakefulness and to upregulate orexin gene expression in animal models.[2] However, to the best of our knowledge, the present study is the first to assess orexin-A levels during DIF. The results clearly demonstrate that orexin-A levels were lower at night and higher in the daytime during DIF. Our findings support previous data indicating that DIF does not cause sleepiness.[1,25] A few previous studies that objectively assessed sleepiness indicated that DIF does not increase daytime sleepiness when controlling for the accompanying lifestyle changes that accompany Ramadan.[1] The effects of intermittent fasting on drowsiness during and outside of Ramadan were objectively assessed. In a well-designed study that measured total blink duration (measured using infrared reflectance oculography) and mean reaction time under controlled conditions with fixed light/dark exposure, caloric intake, sleep/wake schedule, and sleep duration, intermittent fasting had no impact on drowsiness or vigilance.[35] Another study objectively assessed daytime sleepiness using the multiple latency test during Ramadan after controlling for potential confounders in 8 healthy volunteers and revealed no difference in sleep latency between the nonfasting BL, intermittent FOR, and during Ramadan.[8]
The importance of the orexin system in vigilance control was realized recently, with the discovery that patients with narcolepsy, a chronic sleep disorder characterized by irresistible bouts of sleep, cataplexy, and dissociated manifestations of rapid eye movement sleep have orexin deficiency.[36] In patients with narcolepsy, the orexin-A level is usually measured in the CSF; however, we were not granted ethical approval to measure orexin-A levels in the CSF in healthy volunteers, because the blood plasma level of orexin-A is a good marker of orexin-A secretion from hypothalamic orexin+ neurons and reflects the activity of these neurons.[15,16]
It has been proposed that mammals, when faced with a negative energy balance due to reduced food intake, respond with increased wakefulness and alertness in order to enhance their ability to find food.[36] A study has shown that, in orexin neuron-ablated mice, there is no fasting-induced arousal, which indicates that orexin neurons are necessary for the adaptive behavioral arousal during fasting.[37] It is thought that innervation of orexin neurons from regions that control metabolism and feeding such as the hypothalamic arcuate nucleus, nucleus tractus solitarius, and ventromedial hypothalamic nucleus is the likely mechanism enabling orexin neurons to react to the peripheral energy balance and stimulate arousal.[36] In addition, orexin neurons interact closely with the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system and are directly activated by corticotropin-releasing hormone and stress.[38] Stimulation of the sympathetic and HPA tone in response to fasting may further induce arousal through the orexin system.[39]
In the current study, the trend of lower orexin-A levels at night and higher levels in the daytime was more obvious during FOR. FOR gives a better reflection of the pure effect of DIF compared to DIF during Ramadan as there are fewer lifestyle changes during FOR. In the current study, while at home during Ramadan, bedtime and rise time were significantly delayed compared to BL, likely because of the delay in the start of work. Orexin-A levels have been shown to be affected by the sleep/wake pattern and the circadian rhythm.[40] During FOR, both factors were under control. Previous studies have shown that, in the free-living environment, there is a delay in the biological clock during Ramadan among Saudi people.[30] This may explain why the differences between daytime and nighttime orexin-A levels were more obvious during FOR than during Ramadan. Therefore, when assessing orexin levels, it is important to control for sleep/wake patterns and circadian rhythms. A major limitation of previous studies that assessed the effects of fasting during Ramadan on neurohormonal changes is the inability to control for lifestyle changes while at home.[1] Even in controlled studies such as the current study, sleep/wake pattern and hence, light exposure changed during Ramadan while at home, which may affect the measured variables, such as orexin-A levels.[1]
The strength of this study is the fact that it is the first to assess the interaction between the recently discovered excitatory neurotransmitter orexin-A and DIF. In addition, the study was conducted under close monitoring and controlled for potential confounders that may affect orexin-A levels. However, the current study has some limitations that need to be addressed. As the participants were objectively monitored under controlled conditions in a short time period, only a relatively small number of volunteers were recruited. Nevertheless, the recruited number is typical in such experimental studies that use objective continuous assessment methods under controlled conditions that must be conducted within a limited time (the month of Ramadan).[23,30,41,42,43,44] Second, this study was conducted among young healthy subjects; hence, the results cannot be generalized to the general population. Third, women were not included, as they have to break their fast while menstruating, and the total study period covered more than one menstrual cycle.
Conclusions
The current assessment of plasma orexin-A levels in young healthy subjects during and before Ramadan revealed that, under controlled conditions of light exposure, meals, and sleep-wake schedules, DIF increases orexin-A levels in the plasma during fasting hours. This finding supports previous studies in humans and animals, which showed that fasting increases alertness. Future larger studies are needed to confirm the current findings.
Financial support and sponsorship
This work was supported by the Strategic Technologies Program of the National Plan for Sciences and Technology and Innovation in the Kingdom of Saudi Arabia (MED511-02-08).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We are extremely grateful to the study participants who took the time from their busy schedules to participate in the study. Without their participation and feedback, this study would not have been possible.
References
- 1.Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017 doi: 10.1007/s11325-017-1473-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Rashotte ME, Pastukhov IF, Poliakov EL, Henderson RP. Vigilance states and body temperature during the circadian cycle in fed and fasted pigeons (Columba livia) Am J Physiol. 1998;275(5 Pt 2):R1690–702. doi: 10.1152/ajpregu.1998.275.5.R1690. [DOI] [PubMed] [Google Scholar]
- 3.Taoudi Benchekroun M, Roky R, Toufiq J, Benaji B, Hakkou F. Epidemiological study: Chronotype and daytime sleepiness before and during Ramadan. Therapie. 1999;54:567–72. [PubMed] [Google Scholar]
- 4.Margolis SA, Reed RL. Effect of religious practices of Ramadan on sleep and perceived sleepiness of medical students. Teach Learn Med. 2004;16:145–9. doi: 10.1207/s15328015tlm1602_5. [DOI] [PubMed] [Google Scholar]
- 5.Bahammam AS. Sleep pattern, daytime sleepiness, and eating habits during the month of Ramadan. Sleep Hypn. 2003;5:165–74. [Google Scholar]
- 6.Bahammam AS. Effect of fasting during Ramadan on sleep architecture, daytime sleepiness and sleep pattern. Sleep Biol Rhythms. 2004;2:135–43. [Google Scholar]
- 7.BaHammam A. Assessment of sleep patterns, daytime sleepiness, and chronotype during Ramadan in fasting and nonfasting individuals. Saudi Med J. 2005;26:616–22. [PubMed] [Google Scholar]
- 8.Bahammam AS, Almushailhi K, Pandi-Perumal SR, Sharif MM. Intermittent fasting during Ramadan: Does it affect sleep? J Sleep Res. 2014;23:35–43. doi: 10.1111/jsr.12076. [DOI] [PubMed] [Google Scholar]
- 9.Roky R, Chapotot F, Benchekroun MT, Benaji B, Hakkou F, Elkhalifi H, Buguet A. Daytime sleepiness during Ramadan intermittent fasting: Polysomnographic and quantitative waking EEG study. J Sleep Res. 2003;12:95–101. doi: 10.1046/j.1365-2869.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 10.Bahammam AS, Alaseem AM, Alzakri AA, Sharif MM. The effects of Ramadan fasting on sleep patterns and daytime sleepiness: An objective assessment. J Res Med Sci. 2013;18:127–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Richter C, Woods IG, Schier AF. Neuropeptidergic control of sleep and wakefulness. Annu Rev Neurosci. 2014;37:503–31. doi: 10.1146/annurev-neuro-062111-150447. [DOI] [PubMed] [Google Scholar]
- 12.Kukkonen JP. Physiology of the orexinergic/hypocretinergic system: A revisit in 2012. Am J Physiol Cell Physiol. 2013;304:C2–32. doi: 10.1152/ajpcell.00227.2012. [DOI] [PubMed] [Google Scholar]
- 13.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–23. [PubMed] [Google Scholar]
- 14.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai S, Nishijima T, Takahashi S, Yamauchi K, Arihara Z, Takahashi K. Low plasma orexin-A levels were improved by continuous positive airway pressure treatment in patients with severe obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127:731–7. doi: 10.1378/chest.127.3.731. [DOI] [PubMed] [Google Scholar]
- 16.Strawn JR, Pyne-Geithman GJ, Ekhator NN, Horn PS, Uhde TW, Shutter LA, et al. Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology. 2010;35:1001–7. doi: 10.1016/j.psyneuen.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-de-la-Torre M, Barceló A, Piérola J, Esquinas C, de la Peña M, Durán-Cantolla J, et al. Plasma levels of neuropeptides and metabolic hormones, and sleepiness in obstructive sleep apnea. Respir Med. 2011;105:1954–60. doi: 10.1016/j.rmed.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Marston OJ, Williams RH, Canal MM, Samuels RE, Upton N, Piggins HD. Circadian and dark-pulse activation of orexin/hypocretin neurons. Mol Brain. 2008;1:19. doi: 10.1186/1756-6606-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chieffi S, Messina G, Villano I, Messina A, Esposito M, Monda V, et al. Exercise influence on hippocampal function: Possible involvement of orexin-A. Front Physiol. 2017;8:85. doi: 10.3389/fphys.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 21.Adan A, Almirall H. Horne and Östberg morningness-eveningness questionnaire: A reduced scale. Pers Individ Dif. 1991;12:241–53. [Google Scholar]
- 22.BaHammam AS, Almistehi W, Albatli A, AlShaya S. Distribution of chronotypes in a large sample of young adult Saudis. Ann Saudi Med. 2011;31:183–6. doi: 10.4103/0256-4947.78207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alzoghaibi MA, Pandi-Perumal SR, Sharif MM, BaHammam AS. Diurnal intermittent fasting during Ramadan: The effects on leptin and ghrelin levels. PLoS One. 2014;9:e92214. doi: 10.1371/journal.pone.0092214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeneessier AS, BaHammam AS, Sharif MM, BaHammam SA, Nashwan SZ, Pandi-Perumal SR, et al. The influence of intermittent fasting on the circadian pattern of melatonin while controlling for caloric intake, energy expenditure, light exposure and sleep schedules: A preliminary report. Ann Thorac Med. 2017;12:183–90. doi: 10.4103/atm.ATM_15_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahammam A. Does Ramadan fasting affect sleep? Int J Clin Pract. 2006;60:1631–7. doi: 10.1111/j.1742-1241.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 26.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: An EEG study of sleep. Psychophysiology. 1966;2:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 27.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 28.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, et al. Darien, Illinois: American Academy of Sleep Medicine; 2014. [Last accessed on 2017 Mar 29]. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Ver. 2.1. Available from: http://www.aasmnet.org . [Google Scholar]
- 29.Dorminy CA, Choi L, Akohoue SA, Chen KY, Buchowski MS. Validity of a multisensor armband in estimating 24-h energy expenditure in children. Med Sci Sports Exerc. 2008;40:699–706. doi: 10.1249/MSS.0b013e318161ea8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.BaHammam A, Alrajeh M, Albabtain M, Bahammam S, Sharif M. Circadian pattern of sleep, energy expenditure, and body temperature of young healthy men during the intermittent fasting of Ramadan. Appetite. 2010;54:426–9. doi: 10.1016/j.appet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Malavolti M, Pietrobelli A, Dugoni M, Poli M, Romagnoli E, De Cristofaro P, et al. A new device for measuring resting energy expenditure (REE) in healthy subjects. Nutr Metab Cardiovasc Dis. 2007;17:338–43. doi: 10.1016/j.numecd.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Dorminy CA, Choi L, Akohoue SA, Chen KY, Buchowski MS. Validity of a multisensor armband in estimating 24-h energy expenditure in children. Med Sci Sports Exerc. 2008;40:699–706. doi: 10.1249/MSS.0b013e318161ea8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackey DC, Manini TM, Schoeller DA, Koster A, Glynn NW, Goodpaster BH, et al. Validation of an armband to measure daily energy expenditure in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1108–13. doi: 10.1093/gerona/glr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aksu K, Firat Güven S, Aksu F, Ciftci B, Ulukavak Ciftci T, Aksaray S, et al. Obstructive sleep apnoea, cigarette smoking and plasma orexin-A in a sleep clinic cohort. J Int Med Res. 2009;37:331–40. doi: 10.1177/147323000903700207. [DOI] [PubMed] [Google Scholar]
- 35.Bahammam AS, Nashwan S, Hammad O, Sharif MM, Pandi-Perumal SR. Objective assessment of drowsiness and reaction time during intermittent Ramadan fasting in young men: A case-crossover study. Behav Brain Funct. 2013;9:32. doi: 10.1186/1744-9081-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mieda M. The roles of orexins in sleep/wake regulation. Neurosci Res. 2017;118:56–65. doi: 10.1016/j.neures.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 38.Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): A novel circuit mediating stress response. J Neurosci. 2004;24:11439–48. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmeichel BE, Herman MA, Roberto M, Koob GF. Hypocretin neurotransmission within the central amygdala mediates escalated cocaine self-administration and stress-induced reinstatement in rats. Biol Psychiatry. 2017;81:606–15. doi: 10.1016/j.biopsych.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nattie E, Li A. Respiration and autonomic regulation and orexin. Prog Brain Res. 2012;198:25–46. doi: 10.1016/B978-0-444-59489-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.BaHammam A. Effect of fasting during Ramadan on sleep architecture, daytime sleepiness and sleep pattern. Sleep Biol Rhythms. 2004;2:135–43. [Google Scholar]
- 42.Roky R, Chapotot F, Hakkou F, Benchekroun MT, Buguet A. Sleep during Ramadan intermittent fasting. J Sleep Res. 2001;10:319–27. doi: 10.1046/j.1365-2869.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 43.Bogdan A, Bouchareb B, Touitou Y. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal-time as a synchronizer in humans? Life Sci. 2001;68:1607–15. doi: 10.1016/s0024-3205(01)00966-3. [DOI] [PubMed] [Google Scholar]
- 44.Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. J Biol Rhythms. 2002;17:364–76. doi: 10.1177/074873040201700409. [DOI] [PubMed] [Google Scholar]