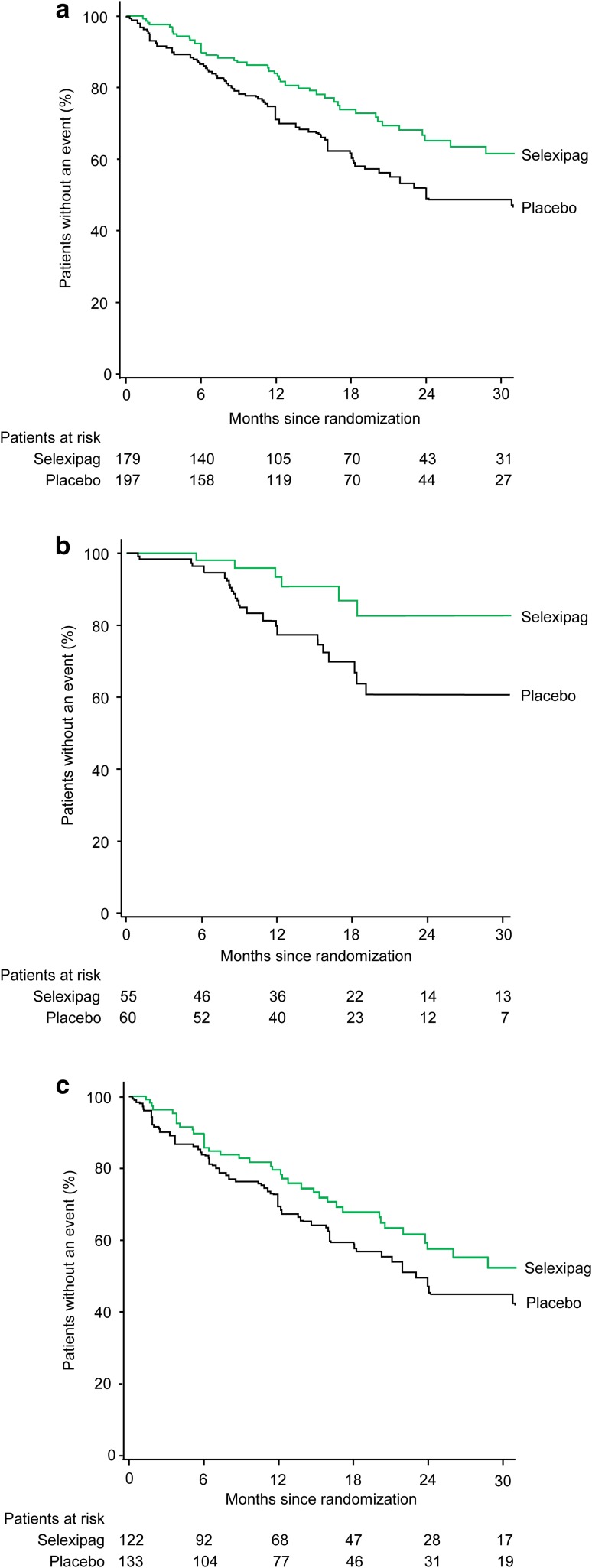
Fig. 1.
Effect of selexipag on the primary composite endpoint of morbidity/mortality up to the end of treatment in patients receiving double combination therapy (with an ERA and PDE-5i) at baseline a overall, b with WHO FC II symptoms at baseline, and c with WHO FC III symptoms at baseline. Kaplan–Meier curves for the primary composite endpoint of morbidity/mortality up to the end of treatment (defined for each patient as 7 days after the date of the last intake of selexipag or placebo) in the selexipag and placebo groups. The hazard ratio (95% confidence interval) for selexipag vs. placebo was 0.63 (0.44–0.90) in the overall group, 0.36 (0.14–0.91) in patients with WHO FC II symptoms, and 0.74 (0.50–1.10) in patients with WHO FC III symptoms. Following adjustment for baseline 6MWD, the hazard ratio (95% confidence interval) was 0.37 (0.15–0.95) in patients with WHO FC II symptoms and 0.67 (0.45–1.01) in patients with WHO FC III symptoms. The analysis considered all available data; the Kaplan–Meier curve is truncated at 30 months because less than 10% of patients are at risk from this time-point onward. 6MWD 6-min walk distance, ERA endothelin receptor antagonist, PDE-5i phosphodiesterase-5 inhibitor, WHO FC World Health Organization functional class