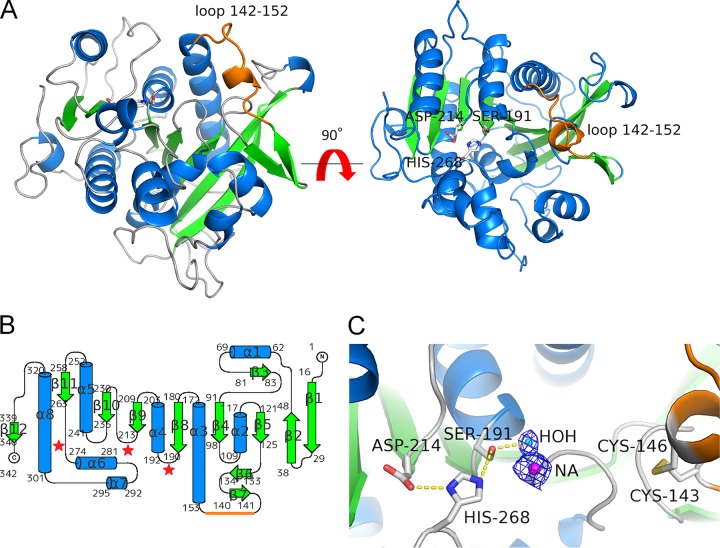
FIG 7.
Crystal structure of rR18. (A) Structure of apo-rR18. Shown are ribbon representations with α-helices, β-strands, and coils marked in cyan, green, and gray, respectively. The catalytic triad residues (Ser-191, Aps-214, and His-268) are represented as sticks, and the loop (residues 142 to 152) is represented as an orange line. (B) Topology diagram of rR18 showing secondary structure elements (α, helices; β, strands). (C) Closeup view of the rR18 active site. The catalytic triad residues and disulfide bridge between Cys-143 and Cys-146 are shown in a stick representation, with the oxygen atom in red, the nitrogen atom in blue, sulfur atoms in yellow, and carbon atoms in white. A sodium ion in the catalytic site of rR18 is represented as NA.