Fig. 4.
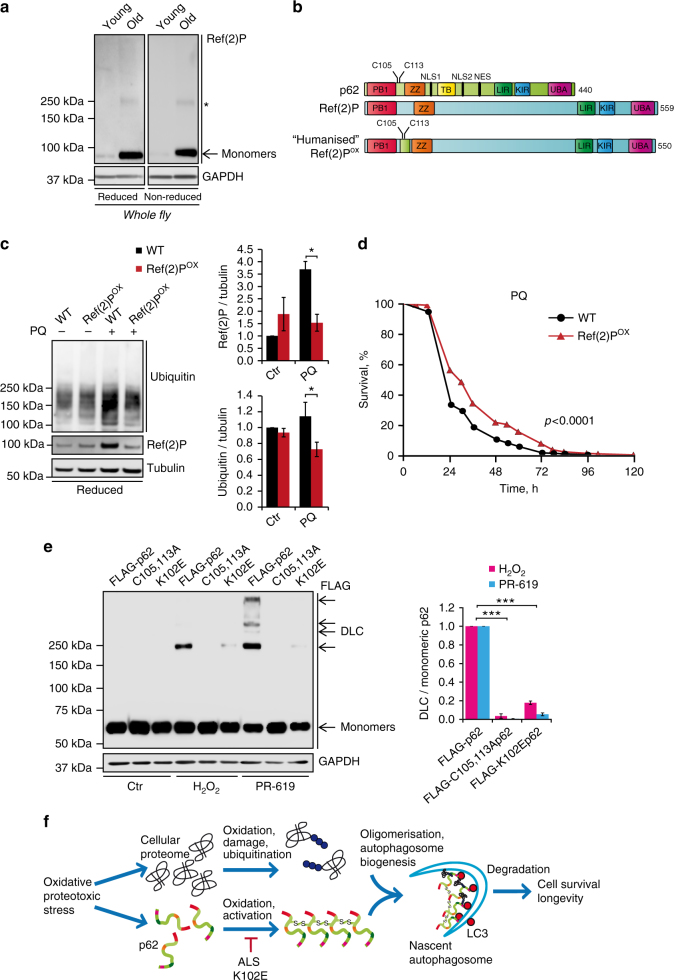
Oxidation-sensitive p62 is important for the oxidative stress resistance of flies and is perturbed in human age-related disease. a Whole-fly lysates were analysed in reducing (2.5% β-ME) and non-reducing conditions for p62 homologue, Ref(2)P. Asterisk indicates a non-specific band. b Diagram representing the introduction of an 18 amino acid fragment of human p62 containing C105 and C113 to produce a ‘humanised’ Ref(2)P (Ref(2)Pox) in flies using CRISPR/Cas9. c Wild-type (WT) and Ref(2)Pox flies were treated with paraquat (PQ, 20 mM) for 12 h and whole-fly lysates were analysed by immunoblot for ubiquitin, Ref(2)P and tubulin and quantified. Error bars represent s.e.m., n = 3 (at least 10 flies per group per replicate); *P < 0.05 (unpaired t-test). d Combined survival data (of three repeats) of wild-type versus Ref(2)Pox flies in the presence of 20 mM PQ. Survival was assessed every 12 h. At least 60 flies per group per replicate were used and log-rank statistics applied. e p62−/− MEFs stably expressing FLAG-tagged wild-type p62, C105A,C113A p62 or ALS-associated mutation K102E p62 were treated with H2O2 (500 μM, 1 min) and PR-619 (20 μM, 10 min), analysed for p62 DLC formation in non-reducing conditions and quantified. Error bars represent s.e.m., n = 3; ***P < 0.005 (unpaired t-tests). f Diagram of the proposed role for p62 oxidation in the aggregation and degradation of autophagy substrates. Formation of p62 DLC is triggered by oxidative stress, which promotes degradation of p62 and bound substrates (e.g. ubiquitylated proteins) through autophagy. Mutations in p62 sequence (e.g. ALS-related K102E) can impair the formation of DLC