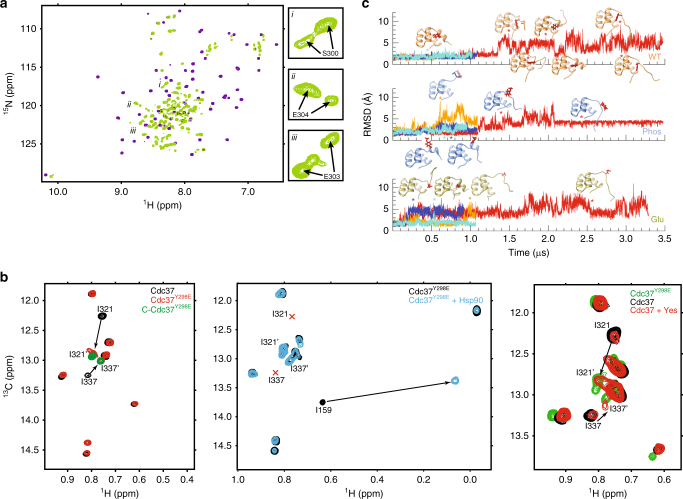
Fig. 2.
Phosphorylation of C-Cdc37 induces partial unfolding. a Overlay of the 15N-HSQC of Y298E (green) and wild-type C-Cdc37 (purple) with selected expansions shown on the right. b Selected regions (Ileδ) from 13C-HMQC spectra of Cdc37 constructs. Left: overlay of full-length wild-type Cdc37 (black), full-length phosphomimetic mutant Cdc37Y298E (red), and C-domain phosphomimetic mutant C-Cdc37Y298E (green). Middle: overlay of free Cdc37Y298E (black) and in the presence of one equivalent deuterated Hsp90α (cyan). The red marks indicate the positions of I321 and I337 signals in wild-type Cdc37. Right: overlay of Cdc37 (black) and Cdc37 in the presence of 15-μg Yes acquired in “phosphorylation” buffer (red), together with Cdc37Y298E (green). c Backbone rmsd for unmodified (top), phosphorylated (middle), and C-Cdc37Y298E (bottom). Simulations at 300 K are shown in dark and light blue, and at 310 K in orange and red. Stars mark the time intervals from which snapshots were extracted