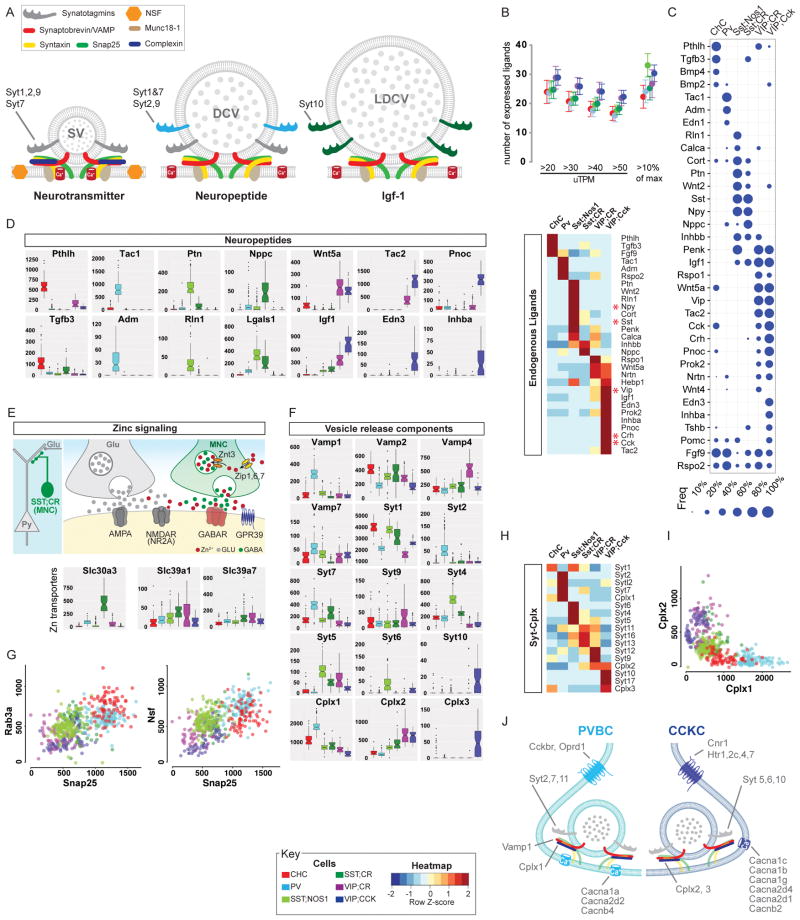
Figure 6. Differential expression of neuropeptides and vesicle release machinery shape outputs and release styles in PCPs.
(A) Schematic of vesicular release machinery for synaptic vesicle (neurotransmitters), dense core vesicle (neuropeptides) and large dense core vesicle (protein/hormones) with putative Syt members.
(B) Top: Each PCP is estimated to express 20–30 peptides based on either a sliding threshold or dynamic threshold (10% of max expression value). Bottom: DE of endogenous ligands that constitute a neuropeptide code for PCPs.
(C) Fraction of individual cells expressing the most common neuropeptides among PCPs. Dot size represents fraction (see key).
(D) ON/OFF expression (uTPM) of specific neuropeptides/endogenous ligands, the gene family that best distinguishes PCPs (AUROC=0.96).
(E) Schematic showing that Zn may be co-released with GABA from SST;CR terminals. While GABA acts on GABAARs, Zn may act on nearby non-synaptic NMDARs and influence glutamatergic transmission. Boxplot: high level specific expression of the Zn vesicular transporter Slc30a3 in SST;CR cells, which also contain the Zn uptake importers Slc39a1 and Slc39a7.
(F) DE of vesicle release machinery components suggest different release styles in Ca2+ sensitivity and dynamics among PCPs.
(G) Scatter plots of mRNA levels (uTPMs) of Snap25 vs Rab3a (left) and Snap25 vs Nsf (right).
(H) Selective expression of Synaptotagmin and Complexin families in PCPs.
(I) Scatterplot of Cplx1 Vs Cplx2 levels shows that fast-release synapses of PV and CHC are biased towards Cplx1 whereas slow-release synapses of CCKCs mainly utilize Cplx2.
(J) Molecular correlates of fast-synchronous and slow-sustained vesicle release mechanisms in PVBC and CCKC with contrasting GABA release styles.
Also see Figure S6.