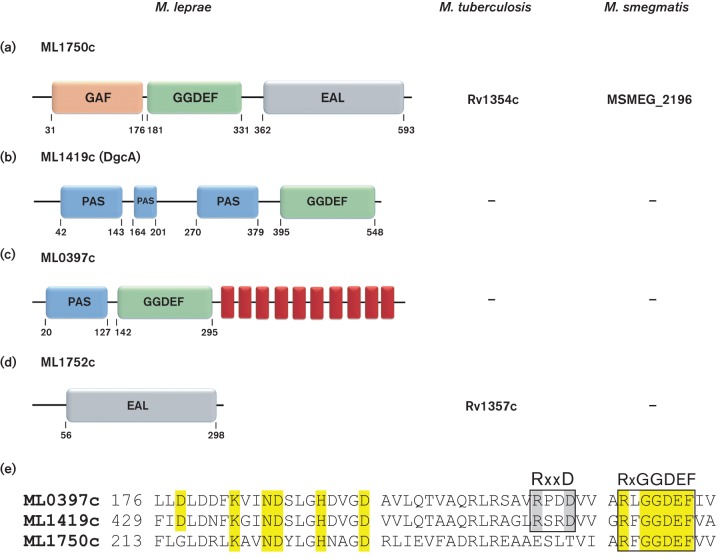
Fig. 1.
Bioinformatics analyses of putative DGC and PDE of M. leprae. (a) ML1750c (623 aa) (gi|15827936|NP_302199) is a hybrid protein containing both GGDEF and EAL motifs and an N-terminal GAF sensory domain. (b) ML1419c (563 aa) (gi|15827746|NP_302009) contains a GGDEF motif and three consecutive PAS sensory domains upstream to GGDEF domain. (c) ML0397c (602 aa) (gi|15827122|NP_301385) possesses a GGDEF motif, an N-terminal PAS sensor domain and 10 transmembrane α-helices (red rectangles). (d) ML1752c (302 aa) (gi|15827938|NP_302201) has a single EAL motif and lacks a sensory domain. Homologues of ML1750c are produced in both M. tuberculosis and M. smegmatis and a homologue of ML1752c is identified in M. tuberculosis. Numbers indicate amino acid positions as reported by CDD NCBI. (e) Alignment of conserved DGC domains of M. leprae proteins ML0397c, ML1419c and ML1750c. The conserved I-site, RxxD motif of ML0397c and ML1419c is highlighted in grey. The conserved A-site, RxGGDEF motif, is present in all proteins. Conserved amino acids involved in enzymatic activity are highlighted in yellow.