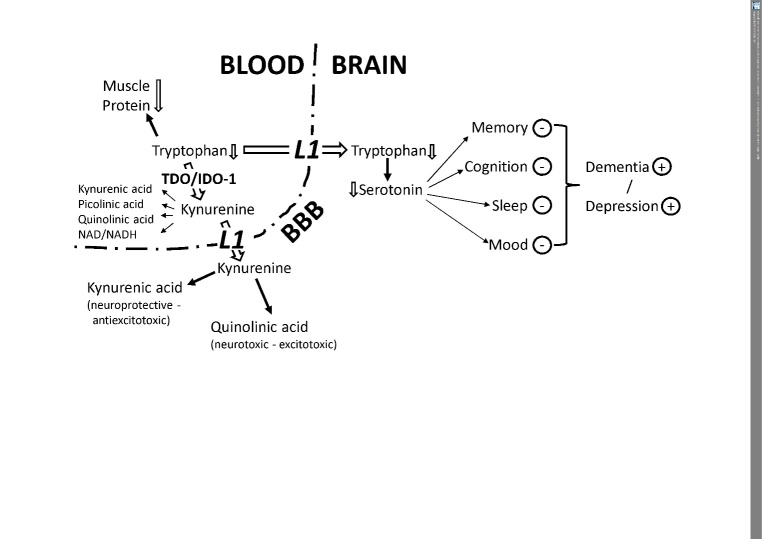
Figure 3. Tryptophan breakdown limits the availability of tryptophan for serotonin synthesis and increases the downstream production of neuroactive metabolites.
Enhanced tryptophan breakdown by the enzymes tryptophan 2,3-dioxygenase (tryptophan pyrrolase, TDO) and/or indoleamine 2,3-dioxygenase-1 (IDO-1) can affect several body compartments including the brain. Thereby, various intermediate catabolites such as kynurenic acid, picolinic acid, and quinolinic acid are formed on the route to nicotinamide adenine dinucleotides. Tryptophan shortage during/after the pro-inflammatory response may reduce the availability of the essential amino acid for the biosynthesis of muscle proteins and can thus contribute to sarcopenia development with older age. For the transport of tryptophan and kynurenine into the brain to cross the blood-brain barrier (BBB), the leucine-preferring L1 system is utilized in competition with the so-called large neutral amino acids (LNAA). Once arrived in the brain, astrocytes are able to convert kynurenine to neuroprotective kynurenic acid, whereas glial cells primarily produce its neurotoxic counterpart quinolinic acid. Alternatively, tryptophan is converted by the tryptophan 5-monooxygenase to 5-hydroxytryptophan, which decarboxylates to the product serotonin (5-hydroxytryptamin), an important neurotransmitter and precursor of the sleep hormone melatonin. If brain tryptophan is low, serotonin also decreases and can disturb memory and cognition as well as sleep and mood, which finally increase the risk of development of dementia and depression.