Figure 2. The mitochondrial carrier system enables redundant substrate utilization.
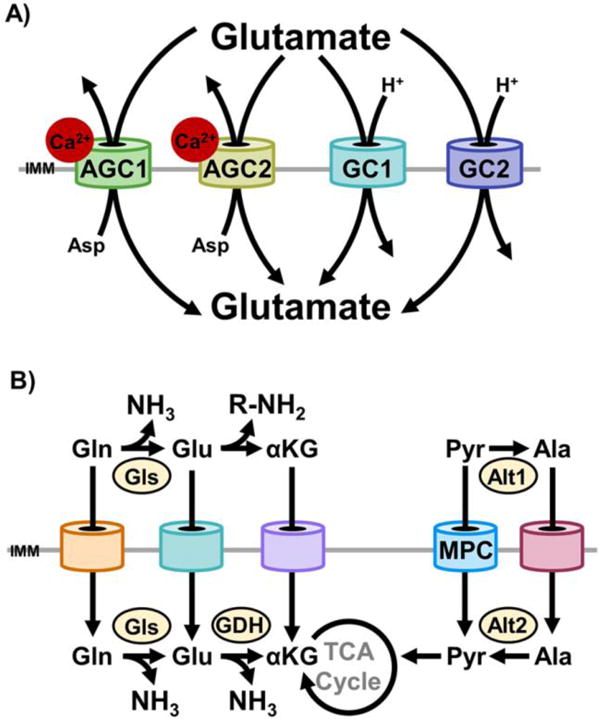
A)AGC1, AGC2, glutamate carrier 1 (GC1), and glutamate carrier 2 (GC2) each transport glutamate into mitochondria. Import through AGC1/2 is in exchange for aspartate export [61]. Import through GC1/2 is by proton (H+) symport [66]. In cells that express each of these carriers, there are at least four distinct portals for glutamate import. Because of differences in co-transported substrates, mechanisms of regulation, and kinetics, functional redundancy is partial rather than absolute. Thus, each mode of glutamate import exerts unique effects on cellular function. B) The presence of distinct mitochondrial carriers for immediate chemical neighbors endows the mitochondrial carrier system with systems-level redundancy. Two examples are illustrated here. In the first, mitochondrial import of glutamine, glutamate, and α-ketoglutarate may all be utilized to anaplerotically replenish the TCA cycle with α-ketoglutarate. However, whether glutamine and glutamate are deaminated in the cytosol or mitochondria changes intracellular nitrogen partitioning. In the second, the MPC and the currently unidentified mitochondrial alanine carrier are functionally redundant. In coordination with the cytosolic (Alt1) and mitochondrial (Alt2) alanine transaminase, either carrier may be utilized to generate mitochondrial pyruvate or alanine. This is exemplified by the pyruvate-alanine cycling employed to bypass disrupted MPC activity [18, 20, 51].
