Fig. 2.
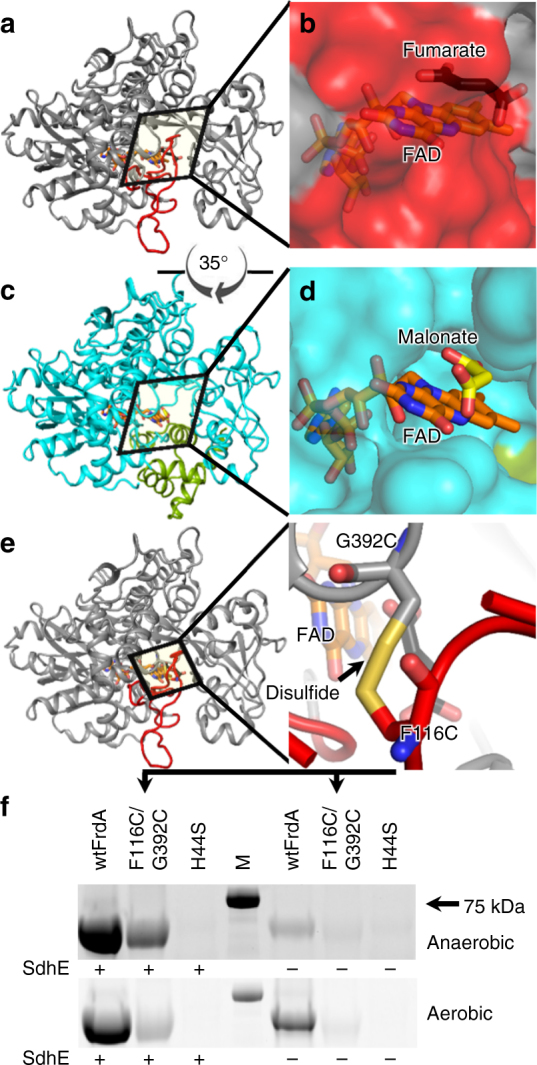
Active site tunnel in the FrdA-SdhE assembly intermediate. a The FrdA subunit within the context of the assembled FrdABCD complex (PDB entry 3P4P21). Residues 50–58 and 103–129 are highlighted in red. b Surface representation of the FrdA subunit within the context of assembled FrdABCD. The surface contributed by the loops (50–58 and 103–129) is shown in red. c The FrdA-SdhE assembly intermediate lacks interpretable electron density for residues 50–58 and 103–129. d Surface representation of the FrdA-SdhE assembly intermediate lacking these loops. e Design of a variant to tether the loop region to the flavin-binding domain using a disulfide (FrdAF116C/G392C, yellow). f Assessment of covalent FAD in wild type, the FrdAF116C/G392C disulfide-trapped variant, and the FrdAH44S negative control, as monitored by measuring FAD fluorescence of equivalent amounts of protein separated by SDS-PAGE. Covalent FAD is anticipated to co-migrate with the polypeptide, while non-covalent FAD would not. Evaluation of the fluorescence of the FrdA subunit therefore reports on covalent flavinylation. Wild-type FrdA subunits have a 1:1 stoichiometry of covalent flavinylation. The FrdAF116C/G392C exhibited reduced covalent flavinylation levels as compared to wild type. Gel is a representative of 12 independent experiments. M molecular weight marker
