Fig. 4.
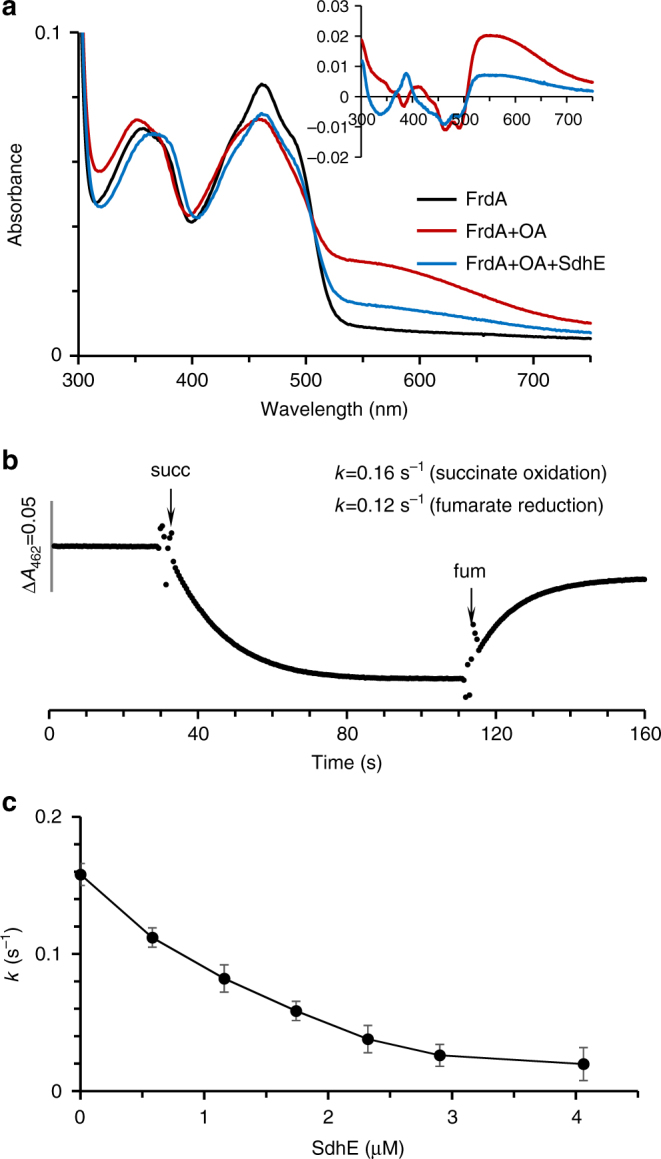
Effect of SdhE on dicarboxylate orientation and succinate oxidase activity of FrdA. a Optical binding spectrum of FrdA and the FrdA-SdhE assembly intermediate with oxaloacetate. The absorbance spectrum of purified, isolated FrdA (9.8 µM) is shown in black. The addition of oxaloacetate (0.2 mM, red line) induces broad spectral changes. As can be seen in the inset, the difference spectrum (FrdA–ligand complex minus free FrdA) exhibits a broad peak characteristic for a charge transfer complex. Addition of SdhE (19.5 µM, blue line) decreases the absorbance of the oxaloacetate-induced charge transfer complex, with significantly reduced charge transfer complex observed in the difference spectrum. b Representative succinate oxidation and fumarate reduction by FrdA subunits (9.8 µM). Absorption at 462 nm corresponds to oxidized FAD. Succinate oxidation was monitored by following the decrease in absorbance of the FAD cofactor at 462 nm upon addition of 5 mM succinate, and fumarate reduction was followed by the increase in absorbance at 462 nm upon addition of 5 mM fumarate, as described in “Methods”. c SdhE inhibition of the succinate-DCIP-reductase reaction catalyzed by FrdA (0.45 µM). The data report the mean of the experiment with the error bars indicative of the variation from experiments. Data in a–c are representative from three or more analyses. All analyses were done at pH 8.0; optical spectra were collected at 25 °C, catalysis was performed at 30 °C
