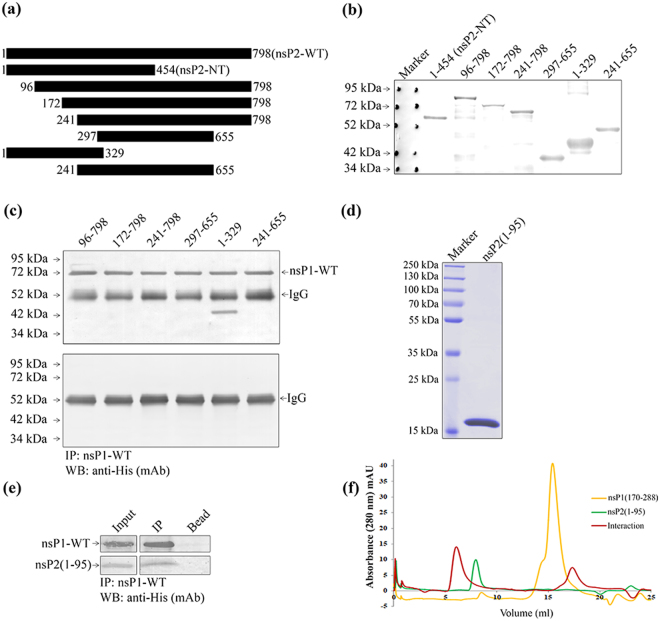
Figure 4.
The residues between 1-95 aa of CHIKV nsP2-NT is responsible for the interaction with nsP1-WT. (a) Graphical representation of different truncations of CHIKV nsP2 indicating specific amino acid positions. (b) The CHIKV nsP2 truncated proteins were over-expressed in BL-21 cells. The Western blot showing the CHIKV nsP2 truncated proteins using anti-His mAb. (c) Both the CHIKV nsP2 truncated and nsP1-WT proteins were incubated at 4o C for 2 hr in vitro for interaction. The protein complexes were immunoprecipitated using anti-nsP1 pAb and separated in 12% SDS-PAGE. The Western blot was probed with anti-His mAb. The lower panel shows the negative control where different nsP2 trancated proteins were immunoprecipitated with nsP1 pAb and beads. (d) Coomassie stained 12% SDS-PAGE showing the purified 1-95 aa fragment of nsP2. (e) The purified 1-95 aa long fragment of nsP2 was incubated with purified nsP1-WT. The protein complex was immunoprecipitated with anti-nsP1 pAb and the Western blot was probed with anti-His mAb. The bead (with nsP1 pAb) was considered as negative control. (f) Analytical size exclusion chromatography was performed using Superdex 200PG 10/300 column with nsP1 (170-288) and nsP2 (1-95) fragments. Chromatogram showing the eluted volume of nsP1 (170-288) (yellow), nsP2 (1-95) (green) and interacted proteins (red) in 280 nm absorbance.