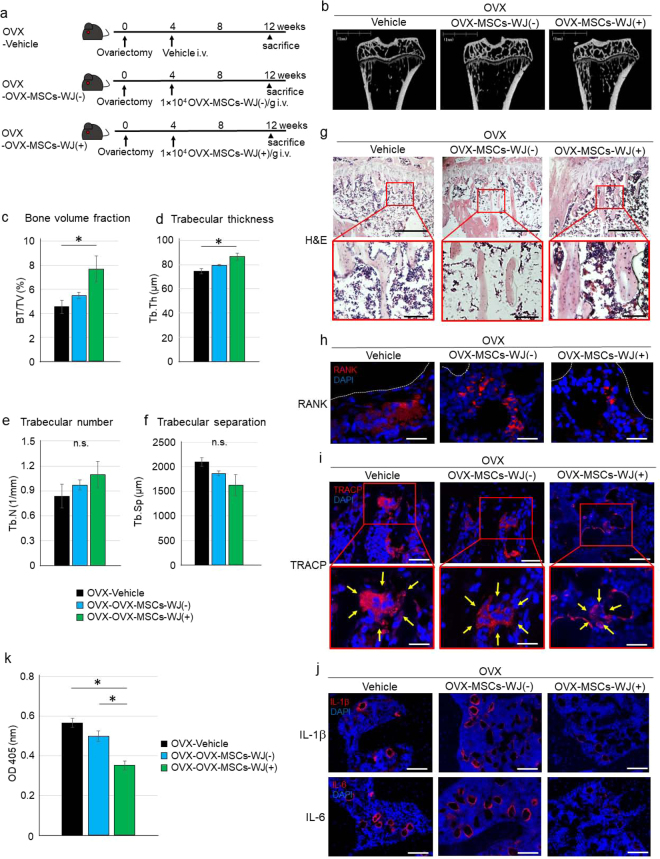
Figure 4.
Therapeutic effect of OVX-MSCs activated with WJS in OVX rats. (a) Experimental protocol for Vehicle, OVX-MSC-WJ(-), and OVX-MSC-WJ(+) therapies in OVX rats. (b) Representative micro-CT images of tibias. (c–f) Quantitative changes in trabecular parameters, including trabecular bone volume, expressed as c: percentage of total tissue volume (BV/TV), d: trabecular thickness (Tb.Th), (e) Trabecular number (Tb.N), and (f) Trabecular separation (Tb.Sp). *P < 0.05. Data are expressed as mean ± SE of 4–5 animals. (g) Histological findings of the tibia in H&E-stained sections at 8 weeks after administration of Vehicle, OVX-MSC-WJ(-), or OVX-MSC-WJ(+) therapies in OVX rats. Bar: 500 μm in upper panel, 100 μm in lower panel. (h) Immunofluorescence staining of the tibia with an anti-RANK antibody (red). DAPI was used for counterstaining nuclei (blue). Bar: 25 µm. (i) Immunofluorescence staining of the tibia with an anti-TRACP antibody (red). DAPI was used for counterstaining nuclei (blue). Bar: upper 50 μm, lower 25 µm. (j) Immunofluorescence staining of the tibia with an anti-IL-1β antibody (red) (upper) and with an anti-IL-6 antibody (red) (lower). DAPI was used for counterstaining nuclei (blue). Bar: 100 μm. (k) Serum TRACP levels at 8 weeks after administration of Vehicle, OVX-MSC-WJ(-), and OVX-MSC-WJ(+) therapies in OVX rats. *P < 0.05. Data are expressed as mean ± SE of 4–5 animals.