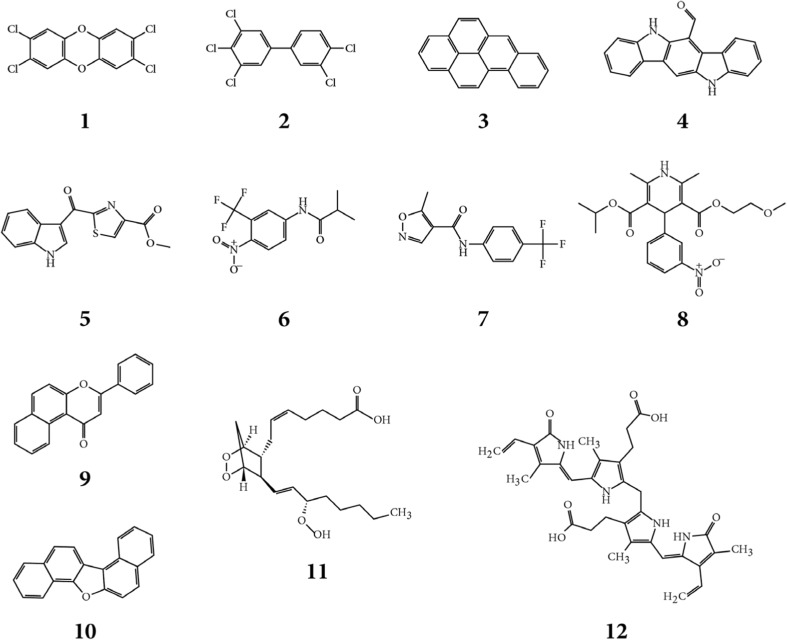
Fig. 1.
Molecular structures of selected compounds that have shown AhR-mediated effects in vitro (AhR modulators, 1–12) and for which some have been shown to competitively bind to AhR (AhR binders, 1–9). Numbers 1, 2, 3, and 9 are examples of typical AhR ligands, i.e., small, rigid, aromatic compounds. Numbers 4, 5, 6, 7, and 8 are atypical AhR ligands, i.e., they are rather flexible aromatic compounds containing additional functional groups and atom types compared to the typical AhR ligands. 1) 2,3,7,8-tetrachloro-dibenzo-p-dioxin (2378-TCDD), 2) 3,3′,4,4′,5-pentachloro-biphenyl (PCB 126), 3) benzo-a-pyrene (BaP), 4) 6-formylindolo[3,2-b]carbazole (FICZ), 5) 2-(1’H-indole-3′-carbonyl)-thiazole-4-carboxylic acid ester (ITE), 6) flutamide, 7) leflunomide, 8) nimodipine, 9) beta-naphthoflavone (BNF), 10) dinaphtho[1,2-b;1′2’-d]furan (DNF), 11) prostaglandin G2, 12) bilirubin