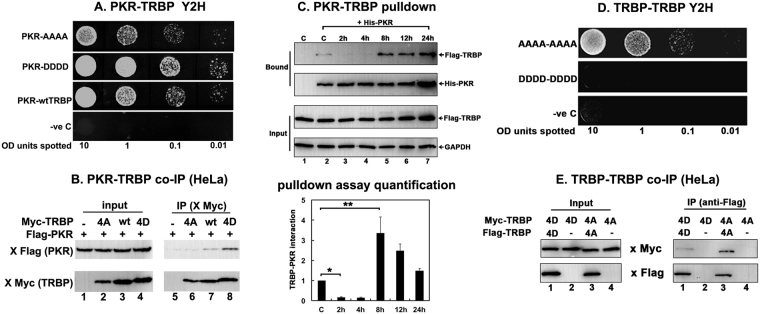
Figure 5.
TRBP phosphorylation strengthens PKR-TRBP interaction and weakens TRBP-TRBP interaction. (A) Phospho-mimic TRBP mutant interacts stronger with PKR compared to the phospho-defective TRBP mutant in yeast two-hybrid assay. PKR/pGAD424 and either AAAA TRBP/pGBKT7, DDDD TRBP/pGBKT7, or wt TRBP/pGBKT7 were co-transformed into AH109 yeast cells and selected on SD double dropout media (-tryptophan, - leucine). Ten microliters of transformed yeast cells (OD600 = 10, 1, 0.1, 0.01) were spotted on SD triple dropout media (-tryptophan, - leucine, - histidine) containing 10 mM 3-amino-1,2,4-triazole (3-AT). Plates were incubated for 3 days at 30 °C. Transformation of PKR in pGAD424 and pGBKT7 empty vector served as a negative control. (B) Phosphomimic TRBP mutant shows stronger heteromeric interaction with PKR compared to the phosphodefective TRBP mutant in mammalian cells. HeLa cells were transfected with Flag K296R PKR/pcDNA 3.1− and either myc TRBP AAAA/pcDNA 3.1−, myc wt TRBP/pcDNA 3.1−, or myc TRBP DDDD/pcDNA 3.1−. The cells were harvested 24 hours after transfection, and myc AAAA, DDDD or wt TRBP was immunoprecipitated using anti-myc monoclonal antibody conjugated agarose beads. Co-immunoprecipitated Flag PKR was analyzed by western blot analysis with an anti-Flag antibody (IP: x Flag (PKR) panel). The blot was subsequently re-probed with anti-myc antibody to ensure equal myc TRBP immunoprecipitation from each sample (IP: x myc (TRBP) panel). Equal Flag PKR and myc TRBP expression in all samples was tested by western blot analysis of equal amounts of total cell lysate with anti-myc, and anti-Flag antibodies (input: x Flag (PKR) and x myc (TRBP) panels). (C) Changes in TRBP association with PKR. Flag TRBP overexpressing cells were treated with 25 μM sodium arsenite for the indicated time points. Cell extracts were prepared in the presence of a phosphatase inhibitor and 25 μg of cell extract was incubated with 500 ng of pure recombinant hexahistidine (His)-tagged PKR immobilized on Ni2+-agarose beads. After washing the beads, PKR-associated Flag TRBP was analyzed by SDS polyacrylamide gel electrophoresis followed by western blot analysis with anti-Flag antibody. Western blot analysis was also performed with anti-His antibody to ensure equal His- PKR in each sample. 25 μg of cell extract was also analyzed by western blot analysis with anti-Flag and anti-GAPDH antibodies to ensure equal addition of cell lysate for each pull down (Input). Quantification of TRBP-PKR pull down: Band intensities were quantified using ImageQuant TL Software, and the ratios of bound TRBP to bound PKR across all samples were calculated and normalized to the band intensities of Flag-TRBP input for each sample. Bound TRBP/his-PKR ratios for all samples were all expressed relative to the control sample (Lane 2). Averages from three independent experiments are plotted as bar graphs ± S.D. One-way ANOVA followed by post-hoc Tukey test was performed, asterisk *p value 0.0000012 and double asterisk **p value 0.0066374. (D) Phosphomimic TRBP mutant shows stronger homomeric interaction compared to the phosphodefective TRBP mutant in yeast two-hybrid assay. AAAA TRBP or DDDD TRBP point mutants in pGADT7 and pGBKT7 were co-transformed into AH109 yeast cells and selected on SD double dropout media (-tryptophan, -leucine). Ten microliters of transformed yeast cells (OD600 = 10, 1, 0.1, 0.01) were spotted on SD triple dropout media plate (tryptophan, -leucine, -histidine) containing 10 mM 3-amino-1,2,4-triazole (3-AT). Plates were incubated for 5 days at 30 °C. Transformation of pGADT7 and pGBKT7 empty vectors served as a negative control. (E) Phosphomimic TRBP mutant shows stronger homomeric interaction compared to the phosphodefective TRBP mutant in mammalian cells. HeLa cells were transfected with either myc TRBP DDDD/pcDNA 3.1− and Flag TRBP DDDD/pcDNA 3.1− or Flag TRBP AAAA/pcDNA 3.1− and myc TRBP AAAA/pcDNA 3.1−. The cells were harvested 24 hours after transfection, and Flag TRBP AAAA or DDDD was immunoprecipitated using anti-Flag monoclonal antibody conjugated agarose beads. The co-immunoprecipitation of myc-TRBP was analyzed by western blot analysis with an anti-myc antibody (IP: x Myc panel). Blot was subsequently stripped and re-probed with anti-Flag antibody to ensure equal Flag-TRBP immunoprecipitation from each sample (IP: x Flag panel). Equal AAAA TRBP and DDDD TRBP expression in all samples was tested by western blot analysis of equal amounts of total cell lysate with anti-myc, and anti-Flag antibodies (Input: x Myc and x Flag panels).