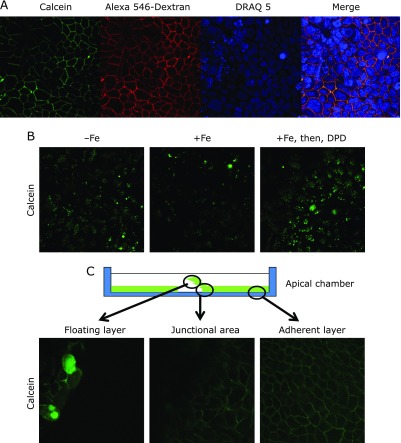
Fig. 2.
Live cell imaging of the Caco-2 monolayer. (A) Uptake of fluorescent probes by endocytosis from basolateral membrane. Nuclei of the Caco-2 monolayer were stained with DRAQ 5 (10 µM) in a CO2 incubator for 30 min. Calcein (200 µM) and Alexa 546-conjugated dextran (M.W. 10,000) (2.5 µM) were added into the basal chamber. After 5 min, confocal images were collected. (B) Dynamic change of calcein after iron feeding and 2,2'-dipyridyl (DPD) addition. In images without Fe, cytoplasmic signals were visualized after extensive washing with HEPES-buffered isotonic solution. In images with Fe, addition of the iron-ascorbate complex (5 µM Fe, 500 µM ascorbate) quenched the fluorescent signals of calcein. In images with Fe, then, DPD (200 µM), after extensive washing with HEPES-buffered isotonic solution, DPD (200 µM) was added into the apical chamber. The green signals derived from calcein were restored. (C) Mechanically loosened monolayer during the uptake of calcein. We scratched the monolayer to break the tight junctions and float the monolayer in the buffer. In the floating layer, a few Caco-2 cells contained calcein probe in the entire cytoplasm; however, the remainder of the Caco-2 monolayer demonstrated basolateral localization. Both the junctional area and adherent layer demonstrated basolateral localization.